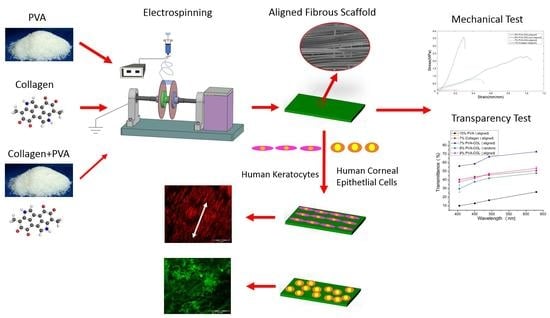
Engineering of Corneal Tissue through an Aligned PVA/Collagen Composite Nanofibrous Electrospun Scaffold
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning
2.3. Crosslinking the Electrospun Fibers for Cell Culture
2.4. SEM Observation and Mechanical Properties
2.5. Light Transmittance
2.6. Cell Culture
2.7. Cell-Seeded Electrospun Scaffolds
2.8. Cell Proliferation Assay
2.9. Observing the Fluorescently Labeled Cells on the Scaffolds
2.10. Statistical Analysis
3. Results
3.1. Morphology of the Electrospun Scaffolds
3.2. Mechanical Properties and the Light Transmittance of the Electrospun Scaffolds
3.3. Proliferation of Cells on the Electrospun Scaffolds
3.4. Fluorescently Labeled HKs on the Electrospun Scaffolds
3.5. Fluorescently Labeled HCECs on the Electrospun Scaffolds
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wilson, S.L.; Wimpenny, I.; Ahearne, M.; Rauz, S.; Haj, A.J.E.; Yang, Y. Chemical and Topographical Effects on Cell Differentiation and Matrix Elasticity in a Corneal Stromal Layer Model. Adv. Funct. Mater. 2012, 22, 3641–3649. [Google Scholar] [CrossRef]
- Mclaughlin, C.R.; Tsai, R.J.; Latorre, M.A.; Griffith, M. Bioengineered corneas for transplantation and in vitro toxicology. Front. Biosci. 2009, 14, 3326–3337. [Google Scholar] [CrossRef]
- Kong, B.; Mi, S. Electrospun Scaffolds for Corneal Tissue Engineering: A Review. Materials 2016, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Draft Action Plan for the Prevention of Avoidable Blindness and Visual Impairment 2014–2019: Towards Universal Eye Health: A Global Action Plan 2014–2019; Report by the Secretariat; Sixty-Sixth World Health Assembly: Geneva, Switzerland, 2013. [Google Scholar]
- Upadhyay, M.P.; Srinivasan, M.; Whitcher, J.P. Corneal blindness: A global perspective. Bull. World Health Organ. 2001, 79, 214–221. [Google Scholar]
- Wu, J.; Du, Y.; Watkins, S.C.; Funderburgh, J.L.; Wagner, W.R. The engineering of organized human corneal tissue through the spatial guidance of corneal stromal stem cells. Biomaterials 2012, 33, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.C.; Patel, S.V.; Hodge, D.O.; Leavitt, J.A. Corneal sensitivity, blink rate, and corneal nerve density in progressive supranuclear palsy and Parkinson disease. Cornea 2013, 32, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Couture, C.; Zaniolo, K.; Carrier, P.; Lake, J.; Patenaude, J.; Germain, L.; Guérin, S.L. The tissue-engineered human cornea as a model to study expression of matrix metalloproteinases during corneal wound healing. Biomaterials 2016, 78, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Fagerholm, P.; Lagali, N.S.; Ong, J.A.; Merrett, K.; Jackson, W.B.; Polarek, J.W.; Suuronen, E.J.; Liu, Y.; Brunette, I.; Griffith, M. Stable corneal regeneration four years after implantation of a cell-free recombinant human collagen scaffold. Biomaterials 2014, 35, 2420–2427. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Choi, J.S.; Wang, Z.; Skardal, A.; Giegengack, M.; Soker, S. Heparin-modified gelatin scaffolds for human corneal endothelial cell transplantation. Biomaterials 2014, 35, 4005–4014. [Google Scholar] [CrossRef] [PubMed]
- Baradaran-Rafii, A.; Biazar, E.; Heidari-Keshel, S. Cellular Response of Limbal Stem Cells on Polycaprolactone Nanofibrous Scaffolds for Ocular Epithelial Regeneration. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 879–887. [Google Scholar] [CrossRef]
- Ye, J.; Shi, X.; Chen, X.; Xie, J.; Wang, C.; Yao, K.; Gao, C.; Gou, Z. Chitosan-modified, collagen-based biomimetic nanofibrous membranes as selective cell adhering wound dressings in the treatment of chemically burned corneas. J. Mater. Chem. B 2014, 2, 4226–4236. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, X.; Lu, T.J.; Xu, F. Tissue Engineering: Recent Advances in Electrospun Nanofibrous Scaffolds for Cardiac Tissue Engineering. Adv. Funct. Mater. 2015, 25, 5726–5738. [Google Scholar] [CrossRef]
- Sahay, R.; Kumar, P.S.; Sridhar, R.; Sundaramurthy, J.; Venugopal, J.; Mhaisalkar, S.G.; Ramakrishna, S. Electrospun composite nanofibers and their multifaceted applications. J. Mater. Chem. 2012, 22, 12953–12971. [Google Scholar] [CrossRef]
- Kong, B.; Sun, W.; Chen, G.; Tang, S.; Li, M.; Shao, Z.; Mi, S. Tissue-engineered cornea constructed with compressed collagen and laser-perforated electrospun mat. Sci. Rep. 2017, 7, 970. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and Gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Parenteau-Bareil, R.; Gauvin, R.; Cliche, S.; Gariépy, C.; Germain, L.; Berthod, F. Comparative study of bovine, porcine and avian collagens for the production of a tissue engineered dermis. Acta Biomater. 2011, 7, 3757–3765. [Google Scholar] [CrossRef] [PubMed]
- Phu, D.; Wray, L.S.; Warren, R.V.; Haskell, R.C.; Orwin, E.J. Effect of substrate composition and alignment on corneal cell phenotype. Tissue Eng. Part A 2011, 17, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Wray, L.S.; Orwin, E.J. Recreating the microenvironment of the native cornea for tissue engineering applications. Tissue Eng. Part A 2009, 15, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.X.; Wang, Y.S.; Ma, C.; Zheng, W.; Li, L.; Zheng, Y.F. Electrospinning of PLGA/gelatin randomly-oriented and aligned nanofibers as potential scaffold in tissue engineering. Mater. Sci. Eng. C 2010, 30, 1204–1210. [Google Scholar] [CrossRef]
- Tang, C.; Saquing, C.D.; Harding, J.R.; Khan, S.A. In Situ Cross-Linking of Electrospun Poly(vinyl alcohol) Nanofibers. Macromolecules 2013, 43, 630–637. [Google Scholar] [CrossRef]
- Nho, Y.C.; Moon, S.W.; Lee, K.H.; Park, C.W.; Suh, T.S.; Jung, Y.J.; Ahn, W.S.; Chun, H.J. Evaluations of poly(vinyl alcohol) hydrogels cross-linked under γ-ray irradiation. J. Ind. Eng. Chem. 2005, 11, 159–164. [Google Scholar]
- Hyon, S.; Cha, W.; Ikada, Y.; Kita, M.; Ogura, Y.; Honda, Y. Poly(vinyl alcohol) hydrogels as soft contact lens material. J. Biomater. Sci. Polym. Ed. 1994, 5, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Toguchida, J.; Oka, M. Preliminary study of polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus. Biomaterials 2003, 24, 639–647. [Google Scholar] [CrossRef]
- Mi, S.; Kong, B.; Wu, Z.; Sun, W.; Xu, Y.; Su, X. A novel electrospinning setup for the fabrication of thickness-controllable 3D scaffolds with an ordered nanofibrous structure. Mater. Lett. 2015, 160, 343–346. [Google Scholar] [CrossRef]
- Acun, A.; Hasirci, V. Construction of a collagen-based, split-thickness cornea substitute. J. Biomater. Sci. Polym. Ed. 2014, 25, 1110–1132. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Shanmuganathan, V.A.; Powellrichards, A.O.; Tighe, P.J.; Joseph, A. Limbal epithelial crypts: A novel anatomical structure and a putative limbal stem cell niche. Br. J. Ophthalmol. 2005, 89, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Yang, X.; Wang, X.; Zhao, M.; Mi, S.; Dou, Z.; Wang, H. Reconstruction of corneal epithelium with cryopreserved corneal limbal stem cells in a rabbit model. Mol. Reprod. Dev. 2008, 75, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Foster, J.; Mi, S.; Chen, B.; Connon, C.J. Influence of substrate on corneal epithelial cell viability within ocular surface models. Exp. Eye Res. 2012, 101, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Connon, C.J.; Kawasaki, S.; Liles, M.; Koizumi, N.; Yamasaki, K.; Nakamura, T.; Quantock, A.J.; Kinoshita, S. Gene expression and immunolocalisation of a calcium-activated chloride channel during the stratification of cultivated and developing corneal epithelium. Cell Tissue Res. 2006, 323, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Moldovan, N.I.; Zhao, Q.; Mi, S.; Zhou, Z.; Chen, D.; Gao, Z.; Tong, D.; Dou, Z. Reconstruction of damaged cornea by autologous transplantation of epidermal adult stem cells. Mol. Vis. 2008, 14, 1064–1070. [Google Scholar] [PubMed]
- Scott, S.G.; Jun, A.S.; Chakravartiabc, S. Sphere formation from corneal keratocytes and phenotype specific markers. Exp. Eye Res. 2011, 93, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Szentmáry, N.; Wang, J.; Stachon, T.; Goebels, S.; Seitz, B. CD34 and alpha-smooth muscle actin expression of keratocytes following photodynamic inactivation (PDI). Klin. Monbl. Augenheilkd. 2013, 230, 570–574. [Google Scholar] [PubMed]
- Lin, H.Y.; Tsai, W.C.; Chang, S.H. Collagen-PVA Aligned Nanofiber on Collagen Sponge as Bi-layered Scaffold for Surface Cartilage Repair. J. Biomater. Sci. Polym. Ed. 2017, 28, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, M.; Zhou, J.; Zheng, W.; Zhou, C.; Dong, D.; Liu, Y.; Teng, Z.; Jiang, Y.; Wei, G. The promotion of neural progenitor cells proliferation by aligned and randomly oriented collagen nanofibers through β1 integrin/MAPK signaling pathway. Biomaterials 2011, 32, 6737–6744. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.; Baek, D.H.; Gang, K.D.; Lee, K.H.; Um, I.C.; Park, Y.H. Characterization of gelatin nanofiber prepared from gelatin–formic acid solution. Polymer 2005, 46, 5094–5102. [Google Scholar]
- Lee, K.H.; Kim, H.Y.; Ryu, Y.J.; Kim, K.W.; Sun, W.C. Mechanical behavior of electrospun fiber mats of poly(vinyl chloride)/polyurethane polyblends. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 1256–1262. [Google Scholar] [CrossRef]
- Torbet, J.; Malbouyres, M.; Builles, N.; Justin, V.; Roulet, M.; Damour, O.; Oldberg, A.; Ruggiero, F.; Hulmes, D.J. Orthogonal scaffold of magnetically aligned collagen lamellae for corneal stroma reconstruction. Biomaterials 2007, 28, 4268–4276. [Google Scholar] [CrossRef] [PubMed]
- Boschetti, F.; Triacca, V.; Spinelli, L.; Pandolfi, A. Mechanical characterization of porcine corneas. J. Biomech. Eng. 2012, 134, 31003. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Qiang, L.; Gao, Y.; Cui, X.; Zhou, H.; Zhong, S.; Wang, Q.; Wang, H. Effect of fiber alignment in electrospun scaffolds on keratocytes and corneal epithelial cells behavior. J. Biomed. Mater. Res. Part A 2012, 100, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Beachley, V.; Hepfer, R.G.; Katsanevakis, E.; Zhang, N.; Wen, X. Precisely Assembled Nanofiber Arrays as a Platform to Engineer Aligned Cell Sheets for Biofabrication. Bioengineering 2014, 1, 114–133. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.N.; Benedek, G.B.; Dohlman, C.H.; Kravitt, B. Structural alterations affecting transparency in swollen human corneas. Investig. Ophthalmol. 1968, 7, 501–519. [Google Scholar]
- Biazar, E.; Baradaran-Rafii, A.; Heidari-Keshel, S.; Tavakolifard, S. Oriented nanofibrous silk as a natural scaffold for ocular epithelial regeneration. J. Biomater. Sci. Polym. Ed. 2015, 26, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Tonsomboon, K.; Oyen, M.L. Composite electrospun gelatin fiber-alginate gel scaffolds for mechanically robust tissue engineered cornea. J. Mech. Behav. Biomed. Mater. 2013, 21, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wen, J.; Yan, J.; Kao, Y.; Ni, Z.; Cui, X.; Wang, H. Growth induction of the corneal stroma cells using uniaxially aligned composite fibrous scaffolds. RSC Adv. 2015, 5, 12123–12130. [Google Scholar] [CrossRef]
- Still, T.J.; Von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar]
- Giugliani, R.; Federhen, A.; Rojas, M.V.; Vieira, T.; Artigalas, O.; Pinto, L.L. Mucopolysaccharidosis I, II, and VI: Brief review and guidelines for treatment. Genet. Mol. Biol. 2010, 33, 589. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.S.; Park, S.H.; Marchant, J.K.; Omenetto, F.G.; Kaplan, D.L. Response of human corneal fibroblasts on silk film surface patterns. Macromol. Biosci. 2010, 10, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.S.; Mandal, B.B.; Park, S.H.; Marchant, J.K.; Omenetto, F.G.; Kaplan, D.L. Helicoidal multi-lamellar features of RGD-functionalized silk biomaterials for corneal tissue engineering. Biomaterials 2010, 31, 8953–8963. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.D.; Marchant, J.K.; Pindrus, M.A.; Omenetto, F.G.; Kaplan, D.L. Silk film biomaterials for cornea tissue engineering. Biomaterials 2009, 30, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Builles, N.; Janin-Manificat, H.; Malbouyres, M.; Justin, V.; Rovere, M.R.; Pellegrini, G. Use of magnetically oriented orthogonal collagen scaffolds for hemi-corneal reconstruction and regeneration. Biomaterials 2010, 31, 8313–8322. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Khutoryanskiy, V.V.; Jones, R.R.; Zhu, X.; Hamley, I.W.; Connon, C.J. Photo-chemical cross-linking of plastically compressed collagen gel produces an optimal scaffold for corneal tissue engineering. J. Biomed. Mater. Res. A 2011, 99, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fagerholm, P.; Lagali, N.S.; Merrett, K.; Jackson, W.B.; Munger, R.; Liu, Y. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci. Transl. Med. 2010, 2, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, H.; Shimmura, S.; Kobayashi, H.; Taguchi, T.; Asano-Kato, N.; Uchino, Y.; Kato, M.; Shimazaki, J.; Tanaka, J.; Tsubota, K. Collagen-immobilized Poly (vinyl alcohol) as an artificial cornea scaffold that supports a stratified corneal epothelium. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 76, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Teixeria, A.I.; Nealey, P.F.; Murphy, C.J. Responses of human keratocytes to micro- and nanostructured substrates. J. Biomed. Mater. Res. A 2004, 71, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Vrana, E.; Builles, N.; Hindie, M.; Damour, O.; Aydinli, A.; Hasirci, V. Contact guidance enhances quality of tissue engineered cornea stroma. J. Biomed. Mater. Res. A 2007, 84, 454–463. [Google Scholar]







| Diameter (nm) | 7% Collagen | 10% PVA | 7% PVA-COL | 9% PVA-COL |
|---|---|---|---|---|
| Non-aligned | 301.5 ± 74.2 | 302 ± 37.9 | 211.6 ± 142.5 | 262.9 ± 199.3 |
| Aligned | 431.8 ± 72.6 | 204 ± 55 | 183.3 ± 96.7 | 163.1 ± 103.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Kong, B.; Liu, R.; Sun, W.; Mi, S. Engineering of Corneal Tissue through an Aligned PVA/Collagen Composite Nanofibrous Electrospun Scaffold. Nanomaterials 2018, 8, 124. https://doi.org/10.3390/nano8020124
Wu Z, Kong B, Liu R, Sun W, Mi S. Engineering of Corneal Tissue through an Aligned PVA/Collagen Composite Nanofibrous Electrospun Scaffold. Nanomaterials. 2018; 8(2):124. https://doi.org/10.3390/nano8020124
Chicago/Turabian StyleWu, Zhengjie, Bin Kong, Rui Liu, Wei Sun, and Shengli Mi. 2018. "Engineering of Corneal Tissue through an Aligned PVA/Collagen Composite Nanofibrous Electrospun Scaffold" Nanomaterials 8, no. 2: 124. https://doi.org/10.3390/nano8020124
APA StyleWu, Z., Kong, B., Liu, R., Sun, W., & Mi, S. (2018). Engineering of Corneal Tissue through an Aligned PVA/Collagen Composite Nanofibrous Electrospun Scaffold. Nanomaterials, 8(2), 124. https://doi.org/10.3390/nano8020124