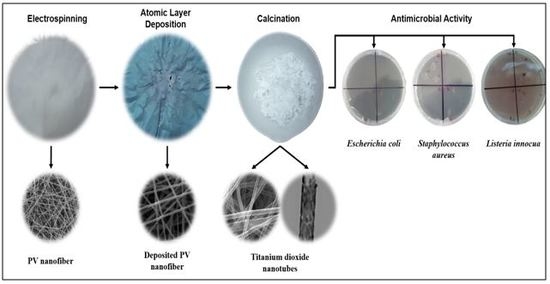
Novel Antimicrobial Titanium Dioxide Nanotubes Obtained through a Combination of Atomic Layer Deposition and Electrospinning Technologies
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.1.1. Polymers, Chemicals and Microorganisms
2.1.2. Electrospun PV Nanofibers
2.1.3. Atomic Layer Deposition Process (ALD)
2.1.4. Polymer Template Removal
2.2. Characterization of Nanostructures: Nanofibers and Nanotubes
2.2.1. Electron Microscopy (SEM and TEM)
2.2.2. X-ray Diffraction (XRD)
2.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.4. Thermal Properties
2.3. Antimicrobial Activity of TiO2 Hollow Nanotubes
2.4. Statistical Analysis
3. Results and Discussion
3.1. Morphological Results of Nanofibers and Nanotubes
3.2. Thermal Characterization of Nanofibers and Nanotubes
3.3. X-ray Analysis Results
3.4. FTIR Analysis Results
3.5. Antimicrobial Activities Results
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Colavecchio, A.; Cadieux, B.; Lo, A.; Goodridge, L.D. Bacteriophages Contribute to the Spread of Antibiotic Resistance Genes among Foodborne Pathogens of the Enterobacteriaceae Family—A Review. Front. Microbiol. 2017, 8, 1108. [Google Scholar] [CrossRef] [PubMed]
- Shamsizadeh, Z.; Nikaeen, M.; Nasr Esfahani, B.; Mirhoseini, S.H.; Hatamzadeh, M.; Hassanzadeh, A. Detection of antibiotic resistant Acinetobacter baumannii in various hospital environments: Potential sources for transmission of Acinetobacter infections. Environ. Health Prev. Med. 2017, 22, 44. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, Y.; Yan, L.; Peltier, R.; Hui, W.; Yao, X.; Cui, Y.; Chen, X.; Sun, H.; Wang, Z. Interfacial Engineering of Bimetallic Ag/Pt Nanoparticles on Reduced Graphene Oxide Matrix for Enhanced Antimicrobial Activity. ACS Appl. Mater. Interfaces 2016, 8, 8834–8840. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Sui, M.; Zhang, L.; Sheng, L.; Huang, S.; She, L. Synthesis of ZnO coated multi-walled carbon nanotubes and their antibacterial activities. Sci. Total Environ. 2013, 452–453, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Yah, C.S.; Simate, G.S. Nanoparticles as potential new generation broad spectrum antimicrobial agents. Daru 2015, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.C.; de Foggi, C.C.; Teixeira, M.M.; Da Silva, M.D.P.; Assis, M.; Francisco, E.M.; Pimentel, B.N.; Pereira, P.F.; Vergani, C.E.; Machado, A.L.; et al. Mechanism of Antibacterial Activity via Morphology Change of α-AgVO3: Theoretical and Experimental Insights. ACS Appl. Mater. Interfaces 2017, 9, 11472–11481. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Panáček, A.; Kvítek, L.; Prucek, R.; Kolář, M.; Večeřová, R.; Pizúrová, N.; Sharma, V.K.; Nevěčná, T.; Radek, Z. Silver Colloid Nanoparticles: Synthesis, Characterization, and Their Antibacterial Activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver Nanoparticles as Potential Antiviral Agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, L.A.; Zapata, P.A.; Vejar, N.D.; Azócar, M.I.; Gulppi, M.A.; Zhou, X.; Thompson, G.E.; Rabagliati, F.M.; Páez, M.A. Release of silver and copper nanoparticles from polyethylene nanocomposites and their penetration into Listeria monocytogenes. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 40, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Fan, W.; Kishen, A.; Gutmann, J.L.; Fan, B. Evaluation of the Antibacterial Efficacy of Silver Nanoparticles against Enterococcus faecalis Biofilm. J. Endod. 2014, 40, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Greiner, A.; Wendorff, J.H. Functional materials by electrospinning of polymers. Prog. Polym. Sci. 2013, 38, 963–991. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Wang, L.; Ma, G.; Meng, F.; Pritchard, R.H.; Gill, E.L.; Liu, Y.; Huang, Y.Y.S. Low-Voltage Continuous Electrospinning Patterning. ACS Appl. Mater. Interfaces 2016, 8, 32120–32131. [Google Scholar] [CrossRef] [PubMed]
- Marichy, C.; Bechelany, M.; Pinna, N. Atomic Layer Deposition of Nanostructured Materials for Energy and Environmental Applications. Adv. Mater. 2012, 24, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Nielsch, K.; Bachmann, J.; Daub, M.; Jing, J.; Knez, M.; Gösele, U.; Barth, S.; Mathur, S.; Escrig, J.; Altbir, D. Ferromagnetic Nanostructures by Atomic Layer Deposition: From Thin Films Towards Core-Shell Nanotubes. In ECS Transactions; ECS: Wollerau, Switzerland, 2007; Volume 11, pp. 139–148. [Google Scholar]
- Haider, A.; Ozgit-Akgun, C.; Kayaci, F.; Okyay, A.K.; Uyar, T.; Biyikli, N. Fabrication of AlN/BN bishell hollow nanofibers by electrospinning and atomic layer deposition. APL Mater. 2014, 2, 96109. [Google Scholar] [CrossRef] [Green Version]
- Heikkilä, P.; Hirvikorpi, T.; Hilden, H.; Sievänen, J.; Hyvärinen, L.; Harlin, A.; Vähä-Nissi, M. High surface area nanostructured tubes prepared by dissolution of ALD-coated electrospun fibers. J. Mater. Sci. 2012, 47, 3607–3612. [Google Scholar] [CrossRef]
- Peng, Q.; Sun, X.-Y.; Spagnola, J.C.; Hyde, G.K.; Spontak, R.J.; Parsons, G.N. Atomic Layer Deposition on Electrospun Polymer Fibers as a Direct Route to Al2O3 Microtubes with Precise Wall Thickness Control. Nano Lett. 2007, 7, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Borbón-Nuñez, H.A.; Dominguez, D.; Muñoz-Muñoz, F.; Lopez, J.; Romo-Herrera, J.; Soto, G.; Tiznado, H. Fabrication of hollow TiO2 nanotubes through atomic layer deposition and MWCNT templates. Powder Technol. 2017, 308, 249–257. [Google Scholar] [CrossRef]
- Santala, E.; Kemell, M.; Leskelä, M.; Ritala, M. The preparation of reusable magnetic and photocatalytic composite nanofibers by electrospinning and atomic layer deposition. Nanotechnology 2009, 20, 35602. [Google Scholar] [CrossRef] [PubMed]
- Bishal, A.K.; Sukotjo, C.; Takoudis, C.G. Room temperature TiO2 atomic layer deposition on collagen membrane from a titanium alkylamide precursor. J. Vac. Sci. Technol. A Vacuum Surf. Films 2017, 35, 01B134. [Google Scholar] [CrossRef]
- Ghosh, S.; Das, A.P. Modified titanium oxide (TiO2) nanocomposites and its array of applications: A review. Toxicol. Environ. Chem. 2015, 97, 491–514. [Google Scholar] [CrossRef]
- Ma, W.; Li, J.; Liu, Y.; Ren, X.; Gu, Z.-G.; Xie, Z.; Liang, J. Preparation and characterization of excellent antibacterial TiO2/N-halamines nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 284–290. [Google Scholar] [CrossRef]
- Dudefoi, W.; Moniz, K.; Allen-Vercoe, E.; Ropers, M.-H.; Walker, V.K. Impact of food grade and nano-TiO2 particles on a human intestinal community. Food Chem. Toxicol. 2017, 106, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, T.; Nayak, B.; Amirbahman, A.; Tripp, C.P.; Mukhopadhyay, S. Application of ultraviolet light assisted titanium dioxide photocatalysis for food safety: A review. Innov. Food Sci. Emerg. Technol. 2016, 38, 105–115. [Google Scholar] [CrossRef]
- Raut, A.V.; Yadav, H.M.; Gnanamani, A.; Pushpavanam, S.; Pawar, S.H. Synthesis and characterization of chitosan-TiO2:Cu nanocomposite and their enhanced antimicrobial activity with visible light. Colloids Surf. B Biointerfaces 2016, 148, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lv, B.; Wang, Y.; Shen, Q.; Wang, X. Bactericidal mechanisms and effector targets of TiO2 and Ag-TiO2 against Staphylococcus aureus. J. Med. Microbiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Podporska-Carroll, J.; Panaitescu, E.; Quilty, B.; Wang, L.; Menon, L.; Pillai, S.C. Antimicrobial properties of highly efficient photocatalytic TiO2 nanotubes. Appl. Catal. B Environ. 2015, 176–177, 70–75. [Google Scholar] [CrossRef]
- Abendroth, B.; Moebus, T.; Rentrop, S.; Strohmeyer, R.; Vinnichenko, M.; Weling, T.; Stöcker, H.; Meyer, D.C. Atomic layer deposition of TiO2 from tetrakis(dimethylamino)titanium and H2O. Thin Solid Films 2013, 545, 176–182. [Google Scholar] [CrossRef]
- Edy, R.; Zhao, Y.; Huang, G.; Shi, J.; Zhang, J.; Solovev, A.A.; Mei, Y. TiO2 nanosheets synthesized by atomic layer deposition for photocatalysis. Prog. Nat. Sci. Mater. Int. 2016, 26, 493–497. [Google Scholar] [CrossRef]
- Takigawa, T.; Kasihara, H.; Masuda, T. Swelling and mechanical properties of polyvinylalcohol hydrogels. Polym. Bull. 1990, 24, 613–618. [Google Scholar] [CrossRef]
- Mosquera, E.; Rosas, N.; Debut, A.; Guerrero, V.H. Síntesis y Caracterización de Nanopartículas de Dióxido de Titanio Obtenidas por el Método de Sol-Gel. Revista Politécnic 2015, 36, 5. [Google Scholar]
- Park, J.Y.; Choi, S.-W.; Kim, S.S. A synthesis and sensing application of hollow ZnO nanofibers with uniform wall thicknesses grown using polymer templates. Nanotechnology 2010, 21, 475601. [Google Scholar] [CrossRef] [PubMed]
- Shooto, N.D.; Dikio, C.W.; Wankasi, D.; Sikhwivhilu, L.M.; Mtunzi, F.M.; Dikio, E.D. Novel PVA/MOF Nanofibres: Fabrication, Evaluation and Adsorption of Lead Ions from Aqueous Solution. Nanoscale Res. Lett. 2016, 11, 414. [Google Scholar] [CrossRef] [PubMed]
- López de Dicastillo, C.; Garrido, L.; Alvarado, N.; Romero, J.; Palma, J.; Galotto, M. Improvement of Polylactide Properties through Cellulose Nanocrystals Embedded in Poly(Vinyl Alcohol) Electrospun Nanofibers. Nanomaterials 2017, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- López de Dicastillo, C.; Roa, K.; Garrido, L.; Pereira, A.; Galotto, M. Novel Polyvinyl Alcohol/Starch Electrospun Fibers as a Strategy to Disperse Cellulose Nanocrystals into Poly(lactic acid). Polymers 2017, 9, 117. [Google Scholar] [CrossRef]
- Nam, T.; Kim, J.-M.; Kim, M.-K.; Kim, H.; Kim, W.-H. Low-temperature Atomic Layer Deposition of TiO2, Al2O3, and ZnO Thin Films. J. Korean Phys. Soc. 2011, 59, 452–457. [Google Scholar] [CrossRef]
- Xiao, Z.; Guo, P.; Sun, N. Preparation, thermostability, and hydrophobic properties of TiO2/poly(dodecafluoroheptyl methacrylate) nanocomposites. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Mallakpour, S.; Sadeghzadeh, R. Surface modification of alumina with biosafe molecules: Nanostructure, thermal, and mechanical properties of PVA nanocomposites. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Su, L.; Fang, G. Characterization of Cross-linked Alkaline Lignin/Poly(Vinyl Alcohol) Film with a Formaldehyde Cross-linker. BioResources 2014, 9, 4477–4488. [Google Scholar] [CrossRef]
- Guerrini, L.M.; de Oliveira, M.P.; Branciforti, M.C.; Custódio, T.A.; Bretas, R.E.S. Thermal and structural characterization of nanofibers of poly(vinyl alcohol) produced by electrospinning. J. Appl. Polym. Sci. 2009, 112, 1680–1687. [Google Scholar] [CrossRef]
- He, Z.; Cai, Q.; Fang, H.; Situ, G.; Qiu, J.; Song, S.; Chen, J. Photocatalytic activity of TiO2 containing anatase nanoparticles and rutile nanoflower structure consisting of nanorods. J. Environ. Sci. 2013, 25, 2460–2468. [Google Scholar] [CrossRef]
- Xie, Y.; Heo, S.; Yoo, S.; Ali, G.; Cho, S. Synthesis and Photocatalytic Activity of Anatase TiO2 Nanoparticles-coated Carbon Nanotubes. Nanoscale Res. Lett. 2009, 5, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, Y.; Dong, G.; Jiang, Y.; Wei, L.; Su, T.; Fan, R. Three-dimensional flower-like rutile TiO2 microsphere composed of nanorods: A potential material as light scattering layer for DSSCs. Chem. Res. Chin. Univ. 2017, 33, 298–304. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Yu, J. Synthesis of 1-D porous TiO2 on fly ash carriers through surface modification method. J. Phys. Chem. Solids 2017, 107, 7–13. [Google Scholar] [CrossRef]
- Mendoza-Anaya; Salas, P.; Angeles-Chávez, C.; Pérez-Hernández, R.; Castaño, V.M. Caracterización microestructural y morfología de TiO2 para aplicaciones termoluminiscentes. Rev. Mex. Física 2003, 50, 12–16. [Google Scholar]
- Zhuiykov, S.; Akbari, M.K.; Hai, Z.; Xue, C.; Xu, H.; Hyde, L. Wafer-scale fabrication of conformal atomic-layered TiO2 by atomic layer deposition using tetrakis (dimethylamino) titanium and H2O precursors. Mater. Des. 2017, 120, 99–108. [Google Scholar] [CrossRef]
- Fu, G.; Vary, P.S.; Lin, C.-T. Anatase TiO2 Nanocomposites for Antimicrobial Coatings. J. Phys. Chem. B 2005, 109, 8889–8898. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, O.; Mahmoudi Chenari, H.; Ghodsi, F.E.; Ziyadi, H. Preparation of PVA nanofibers containing tungsten oxide nanoparticle by electrospinning and consideration of their structural properties and photocatalytic activity. J. Alloys Compd. 2017, 690, 864–872. [Google Scholar] [CrossRef]
- Enayati, M.S.; Behzad, T.; Sajkiewicz, P.; Bagheri, R.; Ghasemi-Mobarakeh, L.; Kuśnieruk, S.; Rogowska-Tylman, J.; Pahlevanneshan, Z.; Choińska, E.; Święszkowski, W. Fabrication and characterization of electrospun bionanocomposites of poly(vinyl alcohol)/nanohydroxyapatite/cellulose nanofibers. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 660–674. [Google Scholar] [CrossRef]
- Sugiura, K.; Hashimoto, M.; Matsuzawa, S.; Yamaura, K. Influence of degree of crystallinity and syndiotacticity on infrared spectra of solid PVA. J. Appl. Polym. Sci. 2001, 82, 1291–1298. [Google Scholar] [CrossRef]
- Vargas, A.; Ochoa, Y.; Ortegón, Y.; Mosquera, P.; Rodríguez, J.; Amado, R. Nanopartículas de Tio2, fase anatasa, sintetizadas por métodos químicos Nanoparticles of TiO2, anatase phase, synthesized by chemical methods. Ing. Desarro. Univ. Norte 2011, 29, 186–201. [Google Scholar]
- Li, X.; Wang, D.; Luo, Q.; An, J.; Wang, Y.; Cheng, G. Surface modification of titanium dioxide nanoparticles by polyaniline via an in situ method. J. Chem. Technol. Biotechnol. 2008, 83, 1558–1564. [Google Scholar] [CrossRef]
- Pan, H.; Wang, X.; Xiao, S.; Yu, L.; Zhang, Z. Preparation and characterization of TiO2 nanoparticles surface-modified by octadecyltrimethoxysilane. Indian J. Eng. Mater. Sci. 2013, 20, 561–567. [Google Scholar]
- El-Sherbiny, S.; Morsy, F.; Samir, M.; Fouad, O.A. Synthesis, characterization and application of TiO2 nanopowders as special paper coating pigment. Appl. Nanosci. 2014, 4, 305–313. [Google Scholar] [CrossRef]
- León, A.; Reuquen, P.; Garín, C.; Segura, R.; Vargas, P.; Zapata, P.; Orihuela, P. FTIR and Raman Characterization of TiO2 Nanoparticles Coated with Polyethylene Glycol as Carrier for 2-Methoxyestradiol—Semantic Scholar. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.; Jimenez de Aberasturi, D.; Ruiz de Larramendi, I.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, O.K.; Stefanakos, E.; Trotz, M.A.; Goswamia, D.Y. A review of the mechanisms and modeling of photocatalytic disinfection. Appl. Catal. B Environ. 2010, 98, 27–38. [Google Scholar] [CrossRef]
- Dutta, P.K.; Pehkonen, S.O.; Sharma, V.K.; Ray, A.K. Photocatalytic Oxidation of Arsenic(III): Evidence of Hydroxyl Radicals. Environ. Sci. Technol. 2005, 39, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Chu, W. The Hydrogen Peroxide-Assisted Photocatalytic Degradation of Alachlor in TiO2 Suspensions. Environ. Sci. Technol. 2003, 37, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Kambala, V.S.R.; Naidu, R. Disinfection studies on TiO2 thin films prepared by a sol-gel method. J. Biomed. Nanotechnol. 2009, 5, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Gogniat, G.; Dukan, S. TiO2 photocatalysis causes DNA damage via fenton reaction-generated hydroxyl radicals during the recovery period. Appl. Environ. Microbiol. 2007, 73, 7740–7743. [Google Scholar] [CrossRef] [PubMed]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M.V. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Upadhyayula, V.K.K.; Deng, S.; Mitchell, M.C.; Nair, V.K.; Smith, G.B.; Ghoshroy, S. Adsorption kinetics of Escherichia coli and Staphylococcus aureus on single-walled carbon nanotube aggregates. Water Sci. Technol. 2008, 58, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef] [PubMed]
- Erkan, A.; Bakir, U.; Karakas, G. Photocatalytic microbial inactivation over Pd doped SnO2 and TiO2 thin films. J. Photochem. Photobiol. A Chem. 2006, 184, 313–321. [Google Scholar] [CrossRef]
- Ruiz Bolivar, Z.; Poutou Piñales, R.A.; Carrascal Camacho, A.K. Resistencia Antimicrobiana y a Desinfectantes de Spp. Nova 2008, 6, 201. [Google Scholar] [CrossRef]
- Gilbertson, L.M.; Albalghiti, E.M.; Fishman, Z.S.; Perreault, F.; Corredor, C.; Posner, J.D.; Elimelech, M.; Pfefferle, L.D.; Zimmerman, J.B. Shape-Dependent Surface Reactivity and Antimicrobial Activity of Nano-Cupric Oxide. Environ. Sci. Technol. 2016, 50, 3975–3984. [Google Scholar] [CrossRef] [PubMed]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]







| Peaks | PV Polymer | PVN | TiO2 NPs | TDN | Assignment |
|---|---|---|---|---|---|
| a | 3384(a1) | 3380(a1) | 3442(a2) | 3443(a2) | O–H stretching |
| b | 2942 | 2941 | - | - | –CH2– stretching |
| c | 2910 | 2913 | - | - | –CH2– symmetrical and asymmetrical stretching |
| d | 1715 | 1714 | - | - | C=O, C–O band from carbonyl group |
| e | - | - | 1637 | 1639 | bending modes of water Ti–OH |
| f | 1435 | 1434 | - | - | CH2, O–H and C–H bending |
| g | 1377 | 1377 | - | - | CH2 wagging |
| h | 1333 | 1330 | - | - | O–H in-plane bending with C–H wagging |
| i | 1143 | - | - | - | C–C stretching, O–H bending, C–O–C, C–O |
| j | 1094 | 1094 | CO stretching, OCC antisymmetric stretching | ||
| k | 919 | 920 | - | - | CH2 bending |
| l | 851 | 849 | - | - | CH2 rocking |
| m | - | - | 700–400 | 800–400 | Ti–O–Ti bonding |
| Microorganism: | Escherichia coli | Staphylococcus aureus | Listeria innocua | |||
|---|---|---|---|---|---|---|
| TDN (µg/mL) | Cel. conc. (cfu/mL) | Log Reduction | Cel. conc. (cfu /mL) | Log Reduction | Cel. conc. (cfu /mL) | Log Reduction |
| 0 | (3.95 ± 0.16) × 105 | 0 a | (2.02 ± 0.12) × 105 | 0 a | (5.93 ± 0.38) × 105 | 0 a |
| 100 | (5.36 ± 0.29) × 104 | 0.87 ± 0.02 b | (3.14 ± 0.34) × 104 | 0.81 ± 0.04 b | (1.01 ± 0.63) × 105 | 0.84 ± 0.26 b |
| 150 | (8.67 ± 0.47) × 102 | 2.66 ± 0.03 e | (1.27 ± 0.28) × 104 | 1.21 ± 0.08 c | (4.46 ± 0.25) × 104 | 1.12 ± 0.02 c |
| 200 | 0 | 5.59 g | (1.32 ± 0.22) × 103 | 2.19 ± 0.06 d | (4.20 ± 0.27) × 104 | 1.15 ± 0.02 c,d |
| 400 | 0 | 5.59 g | (5.33 ± 0.23) × 102 | 2.58 ± 0.02 e | (5.05 ± 0.07) × 103 | 2.07 ± 0.01 f |
| TiO2 NPs (µg/mL) | Cel. conc. (cfu /mL) | Log Reduction | Cel. conc. (cfu /mL) | Log Reduction | Cel. conc. (cfu /mL) | Log Reduction |
| 0 | (4.58 ± 1.52) × 105 | 0 a | (1.98 ± 0.29) × 105 | 0 a | (3.57 ± 0.48) × 105 | 0 a |
| 100 | (5.42 ± 0.45) × 103 | 1.93 ± 0.04 c | (2.75 ± 0.19) × 102 | 2.86 ± 0.02 f | (1.42 ± 0.21) × 104 | 1.40 ± 0.05 e |
| 150 | (2.77 ± 0.48) × 103 | 2.22 ± 0.08 d | (1.67 ± 0.89) × 102 | 3.12 ± 0.21 g | (1.63 ± 0.65) × 104 | 1.37 ± 0.15 d,e |
| 200 | (5.17 ± 0.88) × 102 | 2.95 ± 0.07 f | 0 | 5.36 h | (1.03 ± 0.12) × 103 | 2.54 ± 0.04 g |
| 400 | 0 | 5.66 g | 0 | 5.36 h | (7.17 ± 0.95) × 102 | 2.69 ± 0.05 g |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López de Dicastillo, C.; Patiño, C.; Galotto, M.J.; Palma, J.L.; Alburquenque, D.; Escrig, J. Novel Antimicrobial Titanium Dioxide Nanotubes Obtained through a Combination of Atomic Layer Deposition and Electrospinning Technologies. Nanomaterials 2018, 8, 128. https://doi.org/10.3390/nano8020128
López de Dicastillo C, Patiño C, Galotto MJ, Palma JL, Alburquenque D, Escrig J. Novel Antimicrobial Titanium Dioxide Nanotubes Obtained through a Combination of Atomic Layer Deposition and Electrospinning Technologies. Nanomaterials. 2018; 8(2):128. https://doi.org/10.3390/nano8020128
Chicago/Turabian StyleLópez de Dicastillo, Carol, Cristian Patiño, María Jose Galotto, Juan Luis Palma, Daniela Alburquenque, and Juan Escrig. 2018. "Novel Antimicrobial Titanium Dioxide Nanotubes Obtained through a Combination of Atomic Layer Deposition and Electrospinning Technologies" Nanomaterials 8, no. 2: 128. https://doi.org/10.3390/nano8020128
APA StyleLópez de Dicastillo, C., Patiño, C., Galotto, M. J., Palma, J. L., Alburquenque, D., & Escrig, J. (2018). Novel Antimicrobial Titanium Dioxide Nanotubes Obtained through a Combination of Atomic Layer Deposition and Electrospinning Technologies. Nanomaterials, 8(2), 128. https://doi.org/10.3390/nano8020128