Kinetics of Ion-Capturing/Ion-Releasing Processes in Liquid Crystal Devices Utilizing Contaminated Nanoparticles and Alignment Films
Abstract
:1. Introduction
2. Theoretical Model and Results
2.1. Contaminated Nanoparticles in Liquid Crystals
2.2. The Effects of Contaminated Alignment Layers
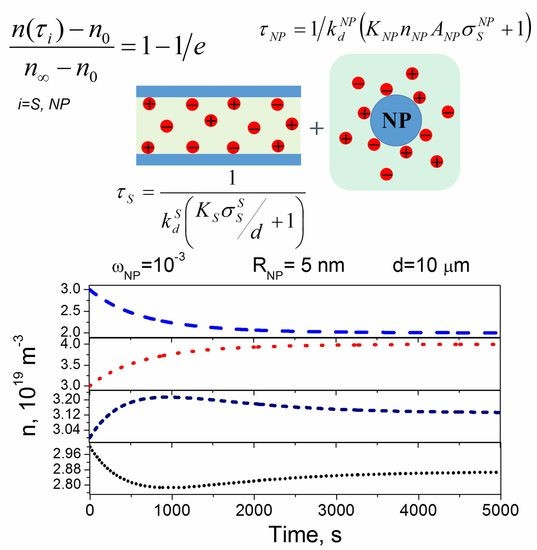
2.3. The Combined Effect of Contaminated Nanoparticles and Substrates
3. Conclusions
Acknowledgments
Conflicts of Interest
References
- Yang, D.-K.; Wu, S.-T. Liquid Crystal Devices; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 1–378. [Google Scholar]
- Chigrinov, V.G. Liquid Crystal Devices: Physics and Applications; Artech House: Boston, MA, USA, 1999; pp. 1–360. [Google Scholar]
- Naemura, S. Electrical properties of liquid crystal materials for display applications. Mater. Res. Soc. Symp. Proc. 1999, 559, 263–274. [Google Scholar] [CrossRef]
- Geis, M.W.; Bos, P.J.; Liberman, V.; Rothschild, M. Broadband optical switch based on liquid crystal dynamic scattering. Opt. Express 2016, 24, 13812–13823. [Google Scholar] [CrossRef] [PubMed]
- Serak, S.V.; Hrozhyk, U.; Hwang, J.; Tabiryan, N.V.; Steeves, D.; Kimball, B.R. High contrast switching of transmission due to electrohydrodynamic effect in stacked thin systems of liquid crystals. Appl. Opt. 2016, 55, 8506–8512. [Google Scholar] [CrossRef] [PubMed]
- Konshina, E.A.; Shcherbinin, D.P. Study of dynamic light scattering in nematic liquid crystal and its optical, electrical and switching characteristics. Liq. Cryst. 2017. [Google Scholar] [CrossRef]
- Garbovskiy, Y.; Glushchenko, I. Nano-objects and ions in liquid crystals: Ion trapping effect and related phenomena. Crystals 2015, 5, 501–533. [Google Scholar] [CrossRef]
- Wu, P.C.; Lisetski, L.N.; Lee, W. Suppressed ionic effect and low-frequency texture transitions in a cholesteric liquid crystal doped with graphene nanoplatelets. Opt. Express 2015, 23, 11195–11204. [Google Scholar] [CrossRef] [PubMed]
- Mun, H.-Y.; Park, H.-G.; Jeong, H.-C.; Lee, J.H.; Oh, B.Y.; Seo, D.-S. Thermal and electro-optical properties of cerium-oxide-doped liquid-crystal devices. Liq. Cryst. 2017, 44, 538–543. [Google Scholar] [CrossRef]
- Shcherbinin, D.P.; Konshina, E.A. Ionic impurities in nematic liquid crystal doped with quantum dots CdSe/ZnS. Liq. Cryst. 2017, 44, 648–655. [Google Scholar] [CrossRef]
- Sharma, K.P.; Malik, P.; Raina, K.K. Electro-optic, dielectric and optical studies of NiFe2O4-ferroelectric liquid crystal: A soft magnetoelectric material. Liq. Cryst. 2016, 43, 1671–1681. [Google Scholar]
- Podgornov, F.V.; Wipf, R.; Stühn, B.; Ryzhkova, A.V.; Haase, W. Low-frequency relaxation modes in ferroelectric liquid crystal/gold nanoparticle dispersion: Impact of nanoparticle shape. Liq. Cryst. 2016, 43, 1536–1547. [Google Scholar] [CrossRef]
- Garbovskiy, Y.; Glushchenko, I. Ion trapping by means of ferroelectric nanoparticles, and the quantification of this process in liquid crystals. Appl. Phys. Lett. 2015, 107, 041106. [Google Scholar] [CrossRef]
- Hsiao, Y.G.; Huang, S.M.; Yeh, E.R.; Lee, W. Temperature-dependent electrical and dielectric properties of nematic liquid crystals doped with ferroelectric particles. Displays 2016, 44, 61–65. [Google Scholar] [CrossRef]
- Garbovskiy, Y. Switching between purification and contamination regimes governed by the ionic purity of nanoparticles dispersed in liquid crystals. Appl. Phys. Lett. 2016, 108, 121104. [Google Scholar] [CrossRef]
- Garbovskiy, Y. Electrical properties of liquid crystal nano-colloids analysed from perspectives of the ionic purity of nano-dopants. Liq. Cryst. 2016, 43, 648–653. [Google Scholar] [CrossRef]
- Tomylko, S.; Yaroshchuk, O.; Kovalchuk, O.; Maschke, U.; Yamaguchi, R. Dielectric properties of nematic liquid crystal modified with diamond nanoparticles. Ukrainian J. Phys. 2012, 57, 239–243. [Google Scholar]
- Samoilov, A.N.; Minenko, S.S.; Fedoryako, A.P.; Lisetski, L.N.; Lebovka, N.I.; Soskin, M.S. Multi-walled vs. single-walled carbon nanotube dispersions in nematic liquid crystals: Comparative studies of optical transmission and dielectric properties. Funct. Mater. 2014, 21, 190–194. [Google Scholar] [CrossRef]
- Yadav, S.P.; Manohar, R.; Singh, S. Effect of TiO2 nanoparticles dispersion on ionic behaviour in nematic liquid crystal. Liq. Cryst. 2015, 42, 1095–1101. [Google Scholar] [CrossRef]
- Garbovskiy, Y. Impact of contaminated nanoparticles on the non-monotonous change in the concentration of mobile ions in liquid crystals. Liq. Cryst. 2016, 43, 664–670. [Google Scholar] [CrossRef]
- Garbovskiy, Y. Adsorption of ions onto nanosolids dispersed in liquid crystals: Towards understanding the ion trapping effect in nanocolloids. Chem. Phys. Lett. 2016, 651, 144–147. [Google Scholar] [CrossRef]
- Urbanski, M.; Lagerwall, J.P.F. Why organically functionalized nanoparticles increase the electrical conductivity of nematic liquid crystal dispersions. J. Mater. Chem. C 2017, 5, 8802–8809. [Google Scholar] [CrossRef]
- Garbovskiy, Y. Nanoparticle enabled thermal control of ions in liquid crystals. Liq. Cryst. 2017, 44, 948–955. [Google Scholar] [CrossRef]
- Garbovskiy, Y. Ions in liquid crystals doped with nanoparticles: Conventional and counterintuitive temperature effects. Liq. Cryst. 2017, 44, 1402–1408. [Google Scholar] [CrossRef]
- Liu, H.; Lee, W. Time-varying ionic properties of a liquid-crystal cell. Appl. Phys. Lett. 2010, 97, 023510. [Google Scholar] [CrossRef]
- Wu, P.-C.; Yang, S.-Y.; Lee, W. Recovery of UV-degraded electrical properties of nematic liquid crystals doped with TiO2 nanoparticles. J. Mol. Liq. 2016, 218, 150–155. [Google Scholar] [CrossRef]
- Takatoh, K.; Hasegawa, M.; Koden, M.; Iton, N.; Hasegawa, R.; Sakamoto, M. Alignment Technologies and Applications of Liquid Crystal Devices; Taylor & Francis: New York, NY, USA, 2005; pp. 1–320. [Google Scholar]
- Furuichi, K.; Xu, J.; Furuta, H.; Kobayashi, S.; Yoshida, N.; Tounai, A.; Tanaka, Y. 38.4: Effect of Ion Capturing Films on the EO Characteristics of Polymer-Stabilized V-FLCD. SID Symp. Dig. Tech. Pap. 2002, 33, 1114–1117. [Google Scholar] [CrossRef]
- Furuichi, K.; Xu, J.; Inoue, M.; Furuta, H.; Yoshida, N.; Tounai, A.; Tanaka, Y.; Mochizuki, A.; Kobayashi, S. Effect of Ion Trapping Films on the Electrooptic Characteristics of Polymer-Stabilized Ferroelectric Liquid Crystal Display Exhibiting V-Shaped Switching. Jpn. J. Appl. Phys. 2003, 42, 4411–4415. [Google Scholar] [CrossRef]
- Kobayashi, S.; Xu, J.; Furuta, H.; Murakami, Y.; Kawamoto, S.; Ohkouchi, M.; Hasebe, H.; Takatsu, H. Fabrication and electro-optic characteristics of polymer-stabilized V-mode ferroelectric liquid crystal display and intrinsic H-V-mode ferroelectric liquid crystal displays: Their application to field sequential full colour active matrix liquid crystal displays. Opt. Eng. 2004, 43, 290–298. [Google Scholar]
- Huang, Y.; Bos, P.J.; Bhowmik, A. The ion capturing effect of 5 SiOx alignment films in liquid crystal devices. J. Appl. Phys. 2010, 108, 064502. [Google Scholar] [CrossRef]
- Huang, Y.; Bhowmik, A.; Bos, P.J. Characterization of Ionic Impurities Adsorbed onto a 5° SiOx Alignment Film. Jpn. J. Appl. Phys. 2012, 51, 031701. [Google Scholar]
- Huang, Y.; Bhowmik, A.; Bos, P.J. The effect of salt on ion adsorption on a SiOx alignment film and reduced conductivity of a liquid crystal host. J. Appl. Phys. 2012, 111, 024501. [Google Scholar] [CrossRef]
- Basu, R.; Lee, A. Ion trapping by the graphene electrode in a graphene-ITO hybrid liquid crystal cell. Appl. Phys. Lett. 2017, 111, 161905. [Google Scholar] [CrossRef]
- Mizusaki, M.; Miyashita, T.; Uchida, T.; Yamada, Y.; Ishii, Y.; Mizushima, S. Generation mechanism of residual direct current voltage in a liquid crystal display and its evaluation parameters related to liquid crystal and alignment layer materials. J. Appl. Phys. 2007, 102, 014904. [Google Scholar] [CrossRef]
- Mizusaki, M.; Miyashita, T.; Uchida, T. Behavior of ion affecting image sticking on liquid crystal displays under application of direct current voltage. J. Appl. Phys. 2010, 108, 104903. [Google Scholar] [CrossRef]
- Mizusaki, M.; Miyashita, T.; Uchida, T. Kinetic analysis of image sticking with adsorption and desorption of ions to a surface of an alignment layer. J. Appl. Phys. 2012, 112, 044510. [Google Scholar] [CrossRef]
- Mizusaki, M.; Yoshimura, Y.; Yamada, Y.; Okamoto, K. Analysis of ion behavior affecting voltage holding property of liquid crystal displays. Jpn. J. Appl. Phys. 2012, 51, 014102. [Google Scholar] [CrossRef]
- Xu, D.; Peng, F.; Chen, H.; Yuan, J.; Wu, S.-T.; Li, M.-C.; Lee, S.-L.; Tsai, W.-C. Image sticking in liquid crystal displays with lateral electric fields. J. Appl. Phys. 2014, 116, 193102. [Google Scholar] [CrossRef]
- Barbero, G.; Evangelista, L.R. Adsorption Phenomena and Anchoring Energy in Nematic Liquid Crystals; Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 1–352. [Google Scholar]
- Garbovskiy, Y. Adsorption/desorption of ions in liquid crystal nano-colloids: The applicability of the Langmuir isotherm, impact of high electric fields, and effects of the nanoparticle’s size. Liq. Cryst. 2016, 43, 853–860. [Google Scholar] [CrossRef]
- Garbovskiy, Y. The purification and contamination of liquid crystals by means of nanoparticles. The case of weakly ionized species. Chem. Phys. Lett. 2016, 658, 331–335. [Google Scholar] [CrossRef]
- Garbovskiy, Y. Ions and size effects in nanoparticle/liquid crystal colloids sandwiched between two substrates. The case of two types of fully ionized species. Chem. Phys. Lett. 2017, 679, 77–85. [Google Scholar] [CrossRef]
- Riley, K.F.; Hobson, M.P.; Bence, S.J. Mathematical Methods for Physics and Engineering; Cambridge University Press: New York, NY, USA, 1997; pp. 1–1008. [Google Scholar]
- Marczewski, A.W. Analysis of kinetic Langmuir model. Part I: Integrated kinetic Langmuir equation (IKL): A new complete analytical solution of the Langmuir rate equation. Langmuir 2010, 26, 15229–15238. [Google Scholar] [CrossRef] [PubMed]
- Gonen, Y.; Rytwo, G. A full analytical solution for the sorption–desorption kinetic process related to Langmuir equilibrium conditions. J. Phys. Chem. C 2007, 111, 1816–1819. [Google Scholar] [CrossRef]
- Tang, C.Y.; Huang, S.M.; Lee, W. Electrical properties of nematic liquid crystals doped with anatase TiO2 nanoparticles. J. Phys. D Appl. Phys. 2011, 44, 355102. [Google Scholar] [CrossRef]
- Jian, B.R.; Tang, C.Y.; Lee, W. Temperature-dependent electrical properties of dilute suspensions of carbon nanotubes in nematic liquid crystals. Carbon 2011, 49, 910–914. [Google Scholar] [CrossRef]
- Wu, P.W.; Lee, W. Phase and dielectric behaviors of a polymorphic liquid crystal doped with graphene nanoplatelets. Appl. Phys. Lett. 2013, 102, 162904. [Google Scholar] [CrossRef]
- Garbovskiy, Y. Ion capturing/ion releasing films and nanoparticles in liquid crystal devices. Appl. Phys. Lett. 2017, 110, 041103. [Google Scholar] [CrossRef]
- Ciuchi, F.; Mazzulla, A.; Pane, A.; Adrian Reyes, J. ac and dc electro-optical response of planar aligned liquid crystal cells. Appl. Phys. Lett. 2007, 91, 232902. [Google Scholar] [CrossRef]




| Materials (Liquid Crystals Doped with Nano-Objects) | Physical Parameters | Ref. |
|---|---|---|
| Nematic liquid crystals (E44) doped with anatase nanoparticles (TiO2) | , , , , | [16,47] |
| Nematic liquid crystals (E7) doped with carbon nanotubes | , , , , | [16,48] |
| Liquid crystals (8OCB) doped with graphene | , , , , | [16,49] |
| Nematic liquid crystals (E44) doped with ferroelectric nanoparticles (BaTiO3) | , , , , | [14,16] |
| Materials (Liquid Crystals/Films) | Physical Parameters | Ref. |
|---|---|---|
| Nematic liquid crystals (ZLI-4792) sandwiched between substrates with alignment layers made of SiOx | , , , | [31] |
| Nematic liquid crystals (ZLI-1132, Merck Corp., Kenilworth, NJ, USA) sandwiched between two substrates with alignment layers (Polyimide AL-1051, JSR Corp, Tokyo, Japan) | , , , | [35] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garbovskiy, Y. Kinetics of Ion-Capturing/Ion-Releasing Processes in Liquid Crystal Devices Utilizing Contaminated Nanoparticles and Alignment Films. Nanomaterials 2018, 8, 59. https://doi.org/10.3390/nano8020059
Garbovskiy Y. Kinetics of Ion-Capturing/Ion-Releasing Processes in Liquid Crystal Devices Utilizing Contaminated Nanoparticles and Alignment Films. Nanomaterials. 2018; 8(2):59. https://doi.org/10.3390/nano8020059
Chicago/Turabian StyleGarbovskiy, Yuriy. 2018. "Kinetics of Ion-Capturing/Ion-Releasing Processes in Liquid Crystal Devices Utilizing Contaminated Nanoparticles and Alignment Films" Nanomaterials 8, no. 2: 59. https://doi.org/10.3390/nano8020059
APA StyleGarbovskiy, Y. (2018). Kinetics of Ion-Capturing/Ion-Releasing Processes in Liquid Crystal Devices Utilizing Contaminated Nanoparticles and Alignment Films. Nanomaterials, 8(2), 59. https://doi.org/10.3390/nano8020059