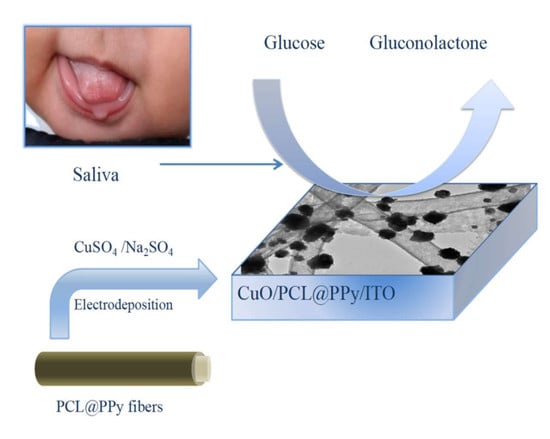
Electrospun CuO-Nanoparticles-Modified Polycaprolactone @Polypyrrole Fibers: An Application to Sensing Glucose in Saliva
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagent
2.2. Instrument and Equipment
2.3. Preparation of Electrospinning PCL Nanofibers
2.4. Preparation of CuO/PCL@PPy/ITO
3. Results and Discussion
3.1. Characterization of as-Prepared Electrodes
3.2. Electrochemical Response of Glucose
3.3. Optimization of Preparation Conditions for CuO/PCL@PPy/ITO Electrodes
3.4. Selectivity and Stability Studies
3.5. Detection of Glucose in Real Samples
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Newman, J.D.; Turner, A.P. Home blood glucose biosensors: A commercial perspective. Biosens. Bioelectron. 2005, 20, 2435–2453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Lei, H.; Feng, L. A facile channel for D-glucose detection in aqueous solution. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 114, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Nery, E.W.; Kundys, M.; Jelen, P.S.; Jonsson-Niedziolka, M. Electrochemical Glucose Sensing: Is There Still Room for Improvement? Anal. Chem. 2016, 88, 11271–11282. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, B.; Choi, C.S.; Lee, K.; Kim, S. Recent trends in the development of diagnostic tools for diabetes mellitus using patient saliva. TrAC Trends Anal. Chem. 2017, 89, 60–67. [Google Scholar] [CrossRef]
- Malik, S.; Khadgawat, R.; Anand, S.; Gupta, S. Non-invasive detection of fasting blood glucose level via electrochemical measurement of saliva. SpringerPlus 2016, 5, 701. [Google Scholar] [CrossRef] [PubMed]
- Elmongy, H.; Abdel-Rehim, M. Saliva as an alternative specimen to plasma for drug bioanalysis: A review. TrAC Trends Anal. Chem. 2016, 83, 70–79. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, W.; Wang, M.L. Sensing of Salivary Glucose Using Nano-Structured Biosensors. Biosensors 2016, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Jha, S.K. A paper strip based non-invasive glucose biosensor for salivary analysis. Biosens Bioelectron. 2015, 67, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sheng, Y.; Sun, Y.; Feng, J.; Wang, S.; Zhang, J.; Xu, J.; Jiang, D. A glucose oxidase-coupled DNAzyme sensor for glucose detection in tears and saliva. Biosens. Bioelectron. 2015, 70, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Fu, Y.; Zhang, W.; Ye, S.; Zhang, H.; Xie, F.; Gong, L.; Wei, Z.; Jin, H.; Chen, J. Highly sensitive detection of glucose: A quantitative approach employing nanorods assembled plasmonic substrate. Talanta 2017, 165, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lu, X.; Zhai, T.; Ling, Y.; Wang, H.; Tong, Y.; Li, Y. Free-standing nickel oxide nanoflake arrays: Synthesis and application for highly sensitive non-enzymatic glucose sensors. Nanoscale 2012, 4, 3123–3127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Su, L.; Zhang, Z.; Huo, D.; Hou, C.; Lei, Y. CuO nanowires based sensitive and selective non-enzymatic glucose detection. Sens. Actuators B Chem. 2014, 191, 86–93. [Google Scholar] [CrossRef]
- Ngo, Y.-L.T.; Hoa, L.T.; Chung, J.S.; Hur, S.H. Multi-dimensional Ag/NiO/reduced graphene oxide nanostructures for a highly sensitive non-enzymatic glucose sensor. J. Alloys Compd. 2017, 712, 742–751. [Google Scholar] [CrossRef]
- Su, Y.; Luo, B.; Zhang, J.Z. Controllable Cobalt Oxide/Au Hierarchically Nanostructured Electrode for Nonenzymatic Glucose Sensing. Anal. Chem. 2016, 88, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, L.; Lu, Y.; Sheng, K.; Liu, W.; Chen, C.; Li, Y.; Dong, B.; Song, H. Engineered IrO2@NiO Core-Shell Nanowires for Sensitive Non-enzymatic Detection of Trace Glucose in Saliva. Anal Chem. 2016, 88, 12346–12353. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Liu, J.; Chen, H.; Gao, Y.; Li, H. Copper/nickel nanoparticle decorated carbon nanotubes for nonenzymatic glucose biosensor. J. Solid State Electrochem. 2015, 19, 1511–1521. [Google Scholar] [CrossRef]
- Gowthaman, N.S.K.; Raj, M.A.; John, S.A. Nitrogen-Doped Graphene as a Robust Scaffold for the Homogeneous Deposition of Copper Nanostructures: A Nonenzymatic Disposable Glucose Sensor. ACS Sustain. Chem. Eng. 2017, 5, 1648–1658. [Google Scholar] [CrossRef]
- Maaoui, H.; Singh, S.K.; Teodorescu, F.; Coffinier, Y.; Barras, A.; Chtourou, R.; Kurungot, S.; Szunerits, S.; Boukherroub, R. Copper oxide supported on three-dimensional ammonia-doped porous reduced graphene oxide prepared through electrophoretic deposition for non-enzymatic glucose sensing. Electrochim. Acta 2017, 224, 346–354. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Guo, M.; Zheng, Z.; Yu, T.; Wang, Y.; Rodriguez, E.G.; Lei, Y. Vertically Aligned CuO Nanowires Based Electrode for Amperometric Detection of Hydrogen Peroxide. Electroanalysis 2008, 20, 2153–2157. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, A.; Wang, G.; Wei, Y.; Wang, W.; Wu, H.; Fang, B. Fabrication of CuO nanowalls on Cu substrate for a high performance enzyme-free glucose sensor. CrystEngComm 2010, 12, 1120–1126. [Google Scholar] [CrossRef]
- Zhuang, Z.; Su, X.; Yuan, H.; Sun, Q.; Xiao, D.; Choi, M.M. An improved sensitivity non-enzymatic glucose sensor based on a CuO nanowire modified Cu electrode. Analyst 2008, 133, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, X.; Liu, G.; Wang, Z.; Wang, F.; Guo, X.; Wen, Y.; Yang, H. Reproducible preparation of a stable polypyrrole-coated-silver nanoparticles decorated polypyrrole-coated-polycaprolactone-nanofiber-based cloth electrode for electrochemical sensor application. Nanotechnology 2015, 26, 445704. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, H.; Shin, Y.M.; Terai, H.; Vacanti, J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Z.G.; Yan, X.; Wang, X.X.; Zhao, H.; Guo, J.; Feng, J.Y.; Long, Y.Z. Chitosan nanostructures by in situ electrospinning for high-efficiency PM 2.5 capture. Nanoscale 2017, 9, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, R.; Lakshminarayanan, R.; Madhaiyan, K.; Barathi, V.A.; Lim, K.H.; Ramakrishna, S. Electrosprayed nanoparticles and electrospun nanofibers based on natural materials: Applications in tissue regeneration, drug delivery and pharmaceuticals. Chem. Soc. Rev. 2015, 44, 790–814. [Google Scholar] [CrossRef] [PubMed]
- Sha, M.; Zhang, H.; Nie, Y.; Nie, K.; Lv, X.; Sun, N.; Xie, X.; Ma, Y.; Sun, X. Sn nanoparticles@nitrogen-doped carbon nanofiber composites as high-performance anodes for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 6277–6283. [Google Scholar] [CrossRef]
- Yadav, K.; Nelson, C.T.; Hsu, S.L.; Hong, Z.; Clarkson, J.D.; Schleputz, C.M.; Damodaran, A.R.; Shafer, P.; Arenholz, E.; Dedon, L.R.; et al. Observation of polar vortices in oxide superlattices. Nature 2016, 530, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Hanson, R.; Kouwenhoven, L.P.; Petta, J.R.; Tarucha, S.; Vandersypen, L.M.K. Spins in few-electron quantum dots. Rev. Mod. Phys. 2007, 79, 1217–1265. [Google Scholar] [CrossRef]
- Du, Y.; Chen, X.; Koh, Y.H.; Lei, B. Facilely fabricating PCL nanofibrous scaffolds with hierarchical pore structure for tissue engineering. Mater. Lett. 2014, 122, 62–65. [Google Scholar] [CrossRef]
- Wei, G.; Li, C.; Fu, Q.; Xu, Y.; Li, H. Preparation of PCL/silk fibroin/collagen electrospun fiber for urethral reconstruction. Int. Urol. Nephrol. 2015, 47, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ying, Y.; Li, L.; Xu, T.; Wu, Y.; Guo, X.; Wang, F.; Shen, H.; Wen, Y.; Yang, H. Stretched graphene tented by polycaprolactone and polypyrrole net–bracket for neurotransmitter detection. Appl. Surf. Sci. 2017, 396, 832–840. [Google Scholar] [CrossRef]
- Saeed, K.; Park, S.-Y.; Lee, H.-J.; Baek, J.-B.; Huh, W.-S. Preparation of electrospun nanofibers of carbon nanotube/polycaprolactone nanocomposite. Polymer 2006, 47, 8019–8025. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Cao, Y.; Li, H. Electrochemical sensor for bisphenol A determination based on MWCNT/melamine complex modified GCE. Sens. Actuators B Chem. 2012, 171–172, 726–733. [Google Scholar] [CrossRef]
- Kang, X.; Mai, Z.; Zou, X.; Cai, P.; Mo, J. A sensitive nonenzymatic glucose sensor in alkaline media with a copper nanocluster/multiwall carbon nanotube-modified glassy carbon electrode. Anal. Biochem. 2007, 363, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, E.; Zhang, X. Non-enzymatic glucose biosensor based on copper oxide-reduced graphene oxide nanocomposites synthesized from water-isopropanol solution. Electrochim. Acta 2014, 130, 253–260. [Google Scholar] [CrossRef]
- Periasamy, P.; Roy, P.; Wu, W.-P.; Huang, Y.-H.; Chang, H.-T. Glucose Oxidase and Horseradish Peroxidase Like Activities of Cuprous Oxide/Polypyrrole Composites. Electrochim. Acta 2016, 215, 253–260. [Google Scholar] [CrossRef]
- Li, X.; Yao, J.; Liu, F.; He, H.; Zhou, M.; Mao, N.; Xiao, P.; Zhang, Y. Nickel/Copper nanoparticles modified TiO2 nanotubes for non-enzymatic glucose biosensors. Sens. Actuators B Chem. 2013, 181, 501–508. [Google Scholar] [CrossRef]
- Meng, F.; Shi, W.; Sun, Y.; Zhu, X.; Wu, G.; Ruan, C.; Liu, X.; Ge, D. Nonenzymatic biosensor based on Cu(x)O nanoparticles deposited on polypyrrole nanowires for improving detection range. Biosens. Bioelectron. 2013, 42, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Nia, P.M.; Meng, W.P.; Lorestani, F.; Mahmoudian, M.R.; Alias, Y. Electrodeposition of copper oxide/polypyrrole/reduced graphene oxide as a nonenzymatic glucose biosensor. Sens. Actuators B Chem. 2015, 209, 100–108. [Google Scholar]
- Wang, T.; Su, W.; Fu, Y.; Hu, J. Controllably annealed CuO-nanoparticle modified ITO electrodes: Characterisation and electrochemical studies. Appl. Surf. Sci. 2016, 390, 795–803. [Google Scholar] [CrossRef]
- Ghanbari, K.; Babaei, Z. Fabrication and characterization of non-enzymatic glucose sensor based on ternary NiO/CuO/polyaniline nanocomposite. Anal. Biochem. 2016, 498, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Long, M.; Tan, L.; Zhang, Y.; Ouyang, J.; Liu, P.; Tang, A. Helical TiO2 Nanotube Arrays Modified by Cu-Cu2O with Ultrahigh Sensitivity for the Nonenzymatic Electro-oxidation of Glucose. ACS Appl. Mater. Interfaces 2015, 7, 12719–12730. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.D.; Han, Y.; Wang, Y.; Xu, J.; Song, Y.Y. One-Step to Prepare Self-Organized Nanoporous NiO/TiO2 Layers and Its Use in Non-Enzymatic Glucose Sensing. Sci. Rep. 2013, 3, 3323. [Google Scholar] [CrossRef] [PubMed]









| Samples | Added (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Saliva | 0 | 38.87 | 0 | 6.05% |
| 50 | 91.47 | 105.1% | 3.26% | |
| 150 | 197.5 | 105.6% | 5.78% | |
| 250 | 279.8 | 96.36% | 5.80% |
| Modified Electrodes | Detection Limit (μM) | Detection Potential (V) | Linear Range | Reference |
|---|---|---|---|---|
| Au/ammonia-doped-prGO/CuO | 0.25 μM | +0.50 | (0.00025–6 mM) | [18] |
| CuO/rGO | 0.1 μM | +0.4 V | 0.0004–3.3 mM | [37] |
| Cu2O/Ppy LT paper electrodes | NA | 0 | 1–40 mM | [38] |
| Ni-Cu/TiO2 NTs | 5 μM | +0.55 V | 0.01–3.2 mM | [39] |
| CuxO/PPy/Au | 6.2 μM | +0.6 V | 0–8 mM | [40] |
| CuxO/Ppy/rGO/GCE | 0.03 μM | +0.2 V | 0.1–100 mM | [41] |
| CuO/ITO | 0.7 μM | +0.59 V | 0–4.4 mM | [42] |
| NiO/CuO/PANI | 2.0 μM | +0.6 V | 0.02–2.500 mM | [43] |
| Cu−Cu2O/TiO2/Ti electrode | 8.6 μM | +0.65 V | 0.1–2.5 mM | [44] |
| NiO/TiO2 | 1.0 μM | +0.47 V | 0.005–12.1 mM | [45] |
| CuO/PCL@PPy/ITO | 2 μM | +0.7 V | 0.002–6 mM | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Jin, W.; Wang, Z.; Cheng, H.; Huang, X.; Guo, X.; Ying, Y.; Wu, Y.; Wang, F.; Wen, Y.; et al. Electrospun CuO-Nanoparticles-Modified Polycaprolactone @Polypyrrole Fibers: An Application to Sensing Glucose in Saliva. Nanomaterials 2018, 8, 133. https://doi.org/10.3390/nano8030133
Xu T, Jin W, Wang Z, Cheng H, Huang X, Guo X, Ying Y, Wu Y, Wang F, Wen Y, et al. Electrospun CuO-Nanoparticles-Modified Polycaprolactone @Polypyrrole Fibers: An Application to Sensing Glucose in Saliva. Nanomaterials. 2018; 8(3):133. https://doi.org/10.3390/nano8030133
Chicago/Turabian StyleXu, Ting, Wen Jin, Zhenzhen Wang, Haiyan Cheng, Xinhua Huang, Xiaoyu Guo, Ye Ying, Yiping Wu, Feng Wang, Ying Wen, and et al. 2018. "Electrospun CuO-Nanoparticles-Modified Polycaprolactone @Polypyrrole Fibers: An Application to Sensing Glucose in Saliva" Nanomaterials 8, no. 3: 133. https://doi.org/10.3390/nano8030133
APA StyleXu, T., Jin, W., Wang, Z., Cheng, H., Huang, X., Guo, X., Ying, Y., Wu, Y., Wang, F., Wen, Y., & Yang, H. (2018). Electrospun CuO-Nanoparticles-Modified Polycaprolactone @Polypyrrole Fibers: An Application to Sensing Glucose in Saliva. Nanomaterials, 8(3), 133. https://doi.org/10.3390/nano8030133