The Preparation of Graphene Oxide-Silver Nanocomposites: The Effect of Silver Loads on Gram-Positive and Gram-Negative Antibacterial Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural and Morphological Properties of Graphene Oxide (GO) Nanosheets
2.2. Structural and Morphological Properties of GO–SH Particles
2.3. Structural and Morphological Properties of GO-Ag Composites
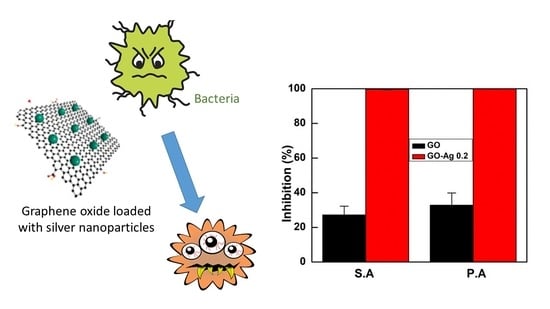
2.4. Antibacterial Results
2.4.1. Antibacterial Activity at a Low Concentration of 10 ppm
2.4.2. Antibacterial Activity at a Concentration of 20 ppm
2.4.3. Antibacterial Activity at a Concentration of 100 ppm
3. Materials and Methods
3.1. Materials
3.2. Bacterial Strains
3.3. Synthesis of GO
3.4. Synthesis of Ag NPs
3.5. Synthesis of GO-Ag NPs
3.6. Characterizations
3.7. Antibacterial Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Garazzino, S.; Lutsar, I.; Bertaina, C.; Tovo, P.-A.; Sharland, M. New antibiotics for paediatric use: A review of a decade of regulatory trials submitted to the European Medicines Agency from 2000—Why aren’t we doing better? Int. J. Antimicrob. Agents 2013, 42, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Graves, J., Jr.; Tajkarimi, M.; Cunningham, Q.; Campbell, A.; Nonga, H.; Harrison, S.; E Barrick, J. Rapid evolution of silver nanoparticle resistance in Escherichia coli. Front. Genet. 2015, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Graves, J.L.; Thomas, M.; Ewunkem, J.A. Antimicrobial nanomaterials: Why evolution matters. Nanomaterials 2017, 7, 283. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Tegou, E.; Magana, M.; Katsogridaki, A.E.; Ioannidis, A.; Raptis, V.; Jordan, S.; Chatzipanagiotou, S.; Chatzandroulis, S.; Ornelas, C.; Tegos, G.P. Terms of endearment: Bacteria meet graphene nanosurfaces. Biomaterials 2016, 89, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Umasuthan, N.; Mohan, R.; Lee, J.; Kim, S.-J. Antibacterial activity of graphene oxide nanosheets. Sci. Adv. Mater. 2012, 4, 1111–1117. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, L.; Wang, Z.; Luo, Y. Mechanisms of the antimicrobial activities of graphene materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef]
- Nanda, S.S.; Yi, D.K.; Kim, K. Study of antibacterial mechanism of graphene oxide using Raman spectroscopy. Sci. Rep. 2016, 6, 28443. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 1–10. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Fernando, K.A.S.; Watson, V.G.; Wang, X.; McNamara, N.D.; JoChum, M.C.; Bair, D.W.; Miller, B.A.; Bunker, C.E. Migration of silver nanoparticles from silver decorated graphene oxide to other carbon nanostructures. Langmuir 2014, 30, 11776–11784. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kuang, D.; Feng, Y.; Zhang, F.; Xu, Z.; Liu, M.; Wang, D. Green synthesis of silver nanoparticles-graphene oxide nanocomposite and its application in electrochemical sensing oftryptophan. Biosens. Bioelectron. 2013, 42, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-C.; Chen, D.-H. Microwave-assisted green synthesis of Ag/reduced graphene oxide nanocomposite as a surface-enhanced Raman scattering substrate with high uniformity. Nanoscale Res. Lett. 2014, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.; Chen, F.; Zhang, C.; Zhi, X.; Wang, K.; Cui, D. The antifungal activity of graphene oxide–silver nanocomposites. Biomaterials 2013, 34, 3882–3890. [Google Scholar] [CrossRef] [PubMed]
- Das, M.R.; Sarma, R.K.; Borah, S.C.; Kumari, R.; Saikia, R.; Deshmukh, A.B.; Shelke, M.V.; Sengupta, P.; Szunerits, S.; Boukherroub, R. The synthesis of citrate-modified silver nanoparticles in an aqueous suspension of graphene oxide nanosheets and their antibacterial activity. Colloids Surf. B: Biointerfaces 2013, 105, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.S.; Badejo, A.C.; Shao, G.N.; Imran, S.M.; Abbas, N.; Chai, Y.G.; Hussain, M.; Kim, H.T. Sequential repetitive chemical reduction technique to study size-property relationships of graphene attached Ag nanoparticle. Solid State Sci. 2015, 44, 1–9. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, D.; Qi, P. Synthesis and characterization of silver nanoparticle and graphene oxide nanosheet composites as a bactericidal agent for water disinfection. J. Colloid Interface Sci. 2011, 360, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Losasso, C.; Belluco, S.; Cibin, V.; Zavagnin, P.; Mičetić, I.; Gallocchio, F.; Zanella, M.; Bregoli, L.; Biancotto, G.; Ricci, A. Antibacterial activity of silver nanoparticles: Sensitivity of different salmonella serovars. Front. Microbiol. 2014, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Zahed, B.; Hosseini-Monfared, H. A comparative study of silver-graphene oxide nanocomposites as a recyclable catalyst for the aerobic oxidation of benzyl alcohol: Support effect. Appl. Surf. Sci. 2015, 328, 536–547. [Google Scholar] [CrossRef]
- Trivedi, M.V.; Laurence, J.S.; Siahaan, T.J. The role of thiols and disulfides in protein chemical and physical stability. Curr. Protein Pept. Sci. 2009, 10, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Heterobifunctional Crosslinkers. In Bioconjugate Techniques, 3rd ed.; Academic Press: Boston, MA, USA, 2013; pp. 299–339. [Google Scholar]
- He, J.; Zhu, X.; Qi, Z.; Wang, C.; Mao, X.; Zhu, C.; He, Z.; Li, M.; Tang, Z. Killing Dental Pathogens Using Antibacterial Graphene Oxide. ACS Appl. Mater. Interfaces 2015, 7, 5605–5611. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Cushing, S.K.; Li, M.; Huang, F.; Wu, N. Origin of strong excitation wavelength dependent fluorescence of graphene oxide. ACS Nano 2014, 8, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Muller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nano 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Kumarasinghe, A.R.; Samaranayake, L.; Bondino, F.; Magnano, E.; Kottegoda, N.; Carlino, E.; Ratnayake, U.N.; de Alwis, A.A.P.; Karunaratne, V.; Amaratunga, G.A.J. Self-assembled multilayer graphene oxide membrane and carbon nanotubes synthesized using a rare form of natural graphite. J. Phys. Chem. C 2013, 117, 9507–9519. [Google Scholar] [CrossRef]
- Yu, B.; Wang, X.; Xing, W.; Yang, H.; Wang, X.; Song, L.; Hu, Y.; Lo, S. Enhanced thermal and mechanical properties of functionalized graphene/thiol-ene systems by photopolymerization technology. Chem. Eng. J. 2013, 228 (Suppl. C), 318–326. [Google Scholar] [CrossRef]
- Glaser, R.E. (Ed.) Vibration spectroscopy tutorial: Sulfur and phosporus. In Organic Spectroscopy; Missouri, MO, USA, 2010; Available online: https://faculty.missouri.edu/~glaserr/8160f10/A03_Silver.pdf (accessed on 1 March 2018).
- Xu, L.Q.; Yang, W.J.; Neoh, K.-G.; Kang, E.-T.; Fu, G.D. Dopamine-Induced Reduction and Functionalization of Graphene Oxide Nanosheets. Macromolecules 2010, 43, 8336–8339. [Google Scholar] [CrossRef]
- Scientific, T. XPS Interpretation of Sodium; Thermo Scientific: Waltham, MA, USA, 2013. [Google Scholar]
- Shchukarev, A.V.; Korolkov, D.V. XPS study of group IA carbonates. Cent. Eur. J. Chem. 2004, 2, 347–362. [Google Scholar] [CrossRef]
- Kim, J.D.; Yun, H.; Kim, G.C.; Lee, C.W.; Choi, H.C. Antibacterial activity and reusability of CNT-Ag and GO-Ag nanocomposites. Appl. Surf. Sci. 2013, 283, 227–233. [Google Scholar] [CrossRef]
- Lukman, A.I.; Gong, B.; Marjo, C.E.; Roessner, U.; Harris, A.T. Facile synthesis, stabilization, and anti-bacterial performance of discrete Ag nanoparticles using Medicago sativa seed exudates. J. Colloid Interface Sci. 2011, 353, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Feng, J. The effect of sonication treatment of graphene oxide on the mechanical properties of the assembled films. RSC Adv. 2016, 6, 39681–39687. [Google Scholar] [CrossRef]
- Yang, Y.-K.; He, C.-E.; He, W.-J.; Yu, L.-J.; Peng, R.-G.; Xie, X.-L.; Wang, X.-B.; Mai, Y.-W. Reduction of silver nanoparticles onto graphene oxide nanosheets with N,N-dimethylformamide and SERS activities of GO/Ag composites. J. Nanopart. Res. 2011, 13, 5571. [Google Scholar] [CrossRef]
- Das, M.R.; Sarma, R.K.; Saikia, R.; Kale, V.S.; Shelke, M.V.; Sengupta, P. Synthesis of silver nanoparticles in an aqueous suspension of graphene oxide sheets and its antimicrobial activity. Colloids Surf. B Biointerfaces 2011, 83, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Jiang, L.; Liu, J.; Xia, Z.; Wang, S.; Sun, G. Facile synthesis of silver nanoparticles supported on three dimensional graphene oxide/carbon black composite and its application for oxygen reduction reaction. Electrochim. Acta 2014, 135, 168–174. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, G.; Zhang, M.; Guo, S.; Liu, Z.H. Preparation and capacitance performance of Ag–graphene based nanocomposite. J. Power Sources 2012, 201, 376–381. [Google Scholar] [CrossRef]
- Ahmad, Z.; Afreen, A.; Mehmood, M.; Ali, I.; Asgher, R.; Aziz, M. One-step synthesis of Ag nano-assemblies and study of their antimicrobial activities. J. Nanostruct. Chem. 2015, 5, 325–331. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Palani, T.; Kumar, M.R.; Lee, S.; Choi, H.C. Preparation of reusable Ag-decorated graphene oxide catalysts for decarboxylative cycloaddition. J. Mater. Chem. 2012, 22, 20665–20670. [Google Scholar] [CrossRef]
- Nishimura, S.; Mott, D.; Takagaki, A.; Maenosono, S.; Ebitani, K. Role of base in the formation of silver nanoparticles synthesized using sodium acrylate as a dual reducing and encapsulating agent. Phys. Chem. Chem. Phys. 2011, 13, 9335–9343. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harbor Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Carmel-Harel, O.; Storz, G. Roles of the glutathione- and thioredoxin-dependent reduction systems in the escherichia coli and saccharomyces cerevisiae responses to oxidative stress. Annu. Rev. Microbiol. 2000, 54, 439–461. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, A.; Chandran, P.; Khan, S.S. Biofunctionalized silver nanoparticles: Advances and prospects. Colloids Surf. B Biointerfaces 2013, 105, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Chen, Q.; Xu, L.; Zhang, S.; Feng, L.; Cheng, L.; Xu, H.; Liu, Z.; Peng, R. Graphene oxide–silver nanocomposite as a highly effective antibacterial agent with species-specific mechanisms. ACS Appl. Mater. Interfaces 2013, 5, 3867–3874. [Google Scholar] [CrossRef] [PubMed]
- Renner, L.D.; Weibel, D.B. Physicochemical regulation of biofilm formation. MRS Bull. 2011, 36, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Dimiev, A.M.; Tour, J.M. Mechanism of graphene oxide formation. ACS Nano 2014, 8, 3060–3068. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Markevich, N.; Gertner, I. Comparison among methods for calculating FWHM. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 1989, 283, 72–77. [Google Scholar] [CrossRef]
- Rout, B.; Liu, C.-H.; Wu, W.-C. Enhancement of photodynamic inactivation against Pseudomonas aeruginosa by a nano-carrier approach. Colloids Surf. B Biointerfaces 2016, 140, 472–480. [Google Scholar] [CrossRef] [PubMed]









| Samples | Carbon Bonding | |||
|---|---|---|---|---|
| C–C | C–O/C–S | C=O | O=C–O | |
| GO | 70.7 | 14.1 | 5.1 | 10.1 |
| GO–SH | 70.1 | 12.2 | 4.6 | 13.2 |
| GO-Ag 0.2 M | 72.5 | 11.2 | 4.6 | 11.7 |
| Samples | C | O | Ag |
|---|---|---|---|
| GO-Ag 0.1 M | 49.2 | 18.3 | 32.4 |
| GO-Ag 0.2 M | 38.5 | 17.1 | 44.3 |
| GO-Ag 0.25 M | 21.5 | 16.5 | 62.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vi, T.T.T.; Rajesh Kumar, S.; Rout, B.; Liu, C.-H.; Wong, C.-B.; Chang, C.-W.; Chen, C.-H.; Chen, D.W.; Lue, S.J. The Preparation of Graphene Oxide-Silver Nanocomposites: The Effect of Silver Loads on Gram-Positive and Gram-Negative Antibacterial Activities. Nanomaterials 2018, 8, 163. https://doi.org/10.3390/nano8030163
Vi TTT, Rajesh Kumar S, Rout B, Liu C-H, Wong C-B, Chang C-W, Chen C-H, Chen DW, Lue SJ. The Preparation of Graphene Oxide-Silver Nanocomposites: The Effect of Silver Loads on Gram-Positive and Gram-Negative Antibacterial Activities. Nanomaterials. 2018; 8(3):163. https://doi.org/10.3390/nano8030163
Chicago/Turabian StyleVi, Truong Thi Tuong, Selvaraj Rajesh Kumar, Bishakh Rout, Chi-Hsien Liu, Chak-Bor Wong, Chia-Wei Chang, Chien-Hao Chen, Dave W. Chen, and Shingjiang Jessie Lue. 2018. "The Preparation of Graphene Oxide-Silver Nanocomposites: The Effect of Silver Loads on Gram-Positive and Gram-Negative Antibacterial Activities" Nanomaterials 8, no. 3: 163. https://doi.org/10.3390/nano8030163
APA StyleVi, T. T. T., Rajesh Kumar, S., Rout, B., Liu, C. -H., Wong, C. -B., Chang, C. -W., Chen, C. -H., Chen, D. W., & Lue, S. J. (2018). The Preparation of Graphene Oxide-Silver Nanocomposites: The Effect of Silver Loads on Gram-Positive and Gram-Negative Antibacterial Activities. Nanomaterials, 8(3), 163. https://doi.org/10.3390/nano8030163