Luminescence Mechanism of Carbon Dots by Tailoring Functional Groups for Sensing Fe3+ Ions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of CDs
2.2. Effects of Carbon Core on Luminescence Property
2.3. Effects of Surface States on Luminescence Property
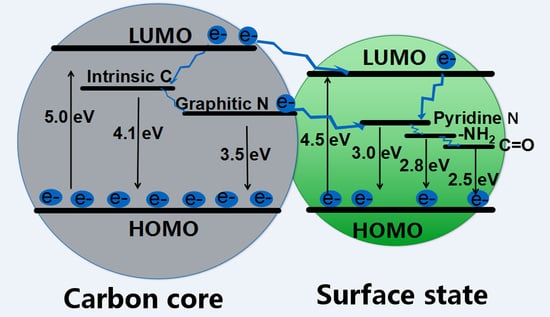
2.4. Theoretical Calculations
2.5. Energy Band Structure of CDs
2.6. Selective Detection of Fe3+ Ions with CDs
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Synthesis of CDs-1, CDs-2, CDs-3, CDs-4, CDs-5, and CDs-6
4.3. Solution of Fe3+ Ions with CDs
4.4. Computational Method
4.5. Characterizations
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, X.M.; Rui, M.C.; Song, J.Z.; Shen, Z.H.; Zeng, H.B. Carbon and Graphene Quantum Dots for Optoelectronic and Energy Devices: A Review. Adv. Funct. Mater. 2015, 25, 4929–4947. [Google Scholar] [CrossRef]
- Briscoe, J.; Marinovic, A.; Sevilla, M.; Dunn, S.; Titirici, M. Biomass-Derived Carbon Quantum Dot Sensitizers for Solid—State Nanostructured Solar Cells. Angew. Chem. Int. Ed. 2015, 54, 4463–4468. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.N.; Zhou, D.; Li, D.; Ji, W.Y.; Jing, P.T.; Han, D.; Liu, L.; Zeng, H.B.; Shen, D.Z. Toward Efficient Orange Emissive Carbon Nanodots through Conjugated sp2-Domain Controlling and Surface Charges Engineering. Adv. Mater. 2016, 28, 3516–3521. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, J.; Cao, Y.; Lu, Y. Facile Access to B-doped Solid-State Fluorescent Carbon Dots toward Light Emitting Devices and Cell Imaging Agents. J. Mater. Chem. C 2015, 3, 6668–6675. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, Green, and Blue Luminescence by Carbon Dots: Full-Color Emission Tuning and Multicolor Cellular Imaging. Angew. Chem. Int. Ed. 2015, 54, 5360–5363. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, Y.; Sun, K.; Reckmeier, C.; Zhang, T.Q.; Zhang, X.Y.; Zhao, J.; Wu, C.F.; Yu, W.W.; Rogach, A.L. Combination of Carbon Dot and Polymer Dot Phosphors for White Light-emitting Diodes. Nanoscale 2015, 7, 12045–12050. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xiang, W.D.; Gao, H.H.; Pei, L.; Ma, X.; Huang, Y.Y.; Liang, X.J. Carbon Dot-Doped Sodium Borosilicate Gel Glasses with Emission Tunability and Their Application in White Light Emitting Diodes. J. Mater. Chem. C 2015, 3, 6764–6770. [Google Scholar] [CrossRef]
- Fang, S.; Xia, Y.; Lv, K.; Li, Q.; Sun, J.; Li, M. Effect of Carbon-dots Modification on the Structure and Photocatalytic Activity of g-C3N4. Appl. Catal. B Environ. 2016, 185, 225–232. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Ma, D.K.; Zhang, Y.G.; Chen, W.; Huang, S.M. N-Doped Carbon Quantum Dots for TiO2-Based Photocatalysts and Dye-Sensitized Solar Cells. Nano Energy 2013, 2, 545–552. [Google Scholar] [CrossRef]
- Feng, T.; Ai, X.Z.; An, G.H.; Yang, P.P.; Zhao, Y.L. Charge-Convertible Carbon Dots for Imaging-Guided Drug Delivery with Enhanced in Vivo Cancer Therapeutic Efficiency. ACS Nano 2016, 10, 4410–4420. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ohulchanskyy, T.Y.; Liu, R.L.; Koynov, K.; Wu, D.Q.; Best, A.; Kumar, R.; Bonoiu, A.; Prasad, P.N. Photoluminescent Carbon Dots as Biocompatible Nanoprobes for Targeting Cancer Cells in Vitro. J. Phys. Chem. C 2010, 114, 12062–12068. [Google Scholar] [CrossRef]
- Miao, P.; Tang, Y.G.; Han, K.; Wang, B.D. Facile Synthesis of Carbon Nanodots from Ethanol and Their Application in Ferric(III) Ion Assay. J. Mater. Chem. A 2015, 3, 15068–15073. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.T.; Yuan, P.; Chi, C.; Zhang, J.; Zhou, N.L. Synthesis of Lanthanum Doped Carbon Dots for Detection of Mercury ion, Multi-color Imaging of Cells and Tissue, and Bacteriostasis. Chem. Eng. J. 2017, 330, 1137–1147. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Y.; Tian, Y.F.; Wang, Y.G.; Yang, W.L. Carbon-Dot-Based Nanosensors for the Detection of Intracellular Redox State. Adv. Mater. 2015, 27, 7156–7160. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Chang, T.; Zhao, H.P.; Du, H.H.; Liu, S.; Wu, B.S.; Qin, S.J. Cultivating Fluorescent Flowers with Highly Luminescent Carbon Dots Fabricated by A Double Passivation Method. Nanomaterials 2017, 7, 176. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.K.; Nandi, S.; Shikler, R.; Jelinek, R. Tuneable Light-Emitting Carbon-Dot/Polymer Flexible Films Prepared through One-Pot Synthesis. Nanoscale 2016, 8, 3400–3406. [Google Scholar] [CrossRef] [PubMed]
- Miao, P.; Han, K.; Tang, Y.G.; Wang, B.D.; Lin, T.; Cheng, W.B. Recent Advances in Carbon Nanodots: Synthesis, Properties and Biomedical Applications. Nanoscale 2015, 7, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, S.J.; Wang, H.Y.; Qu, S.N.; Zhang, Y.L.; Zhang, J.H.; Chen, Q.D.; Xu, H.L.; Han, W.; Yang, B.; et al. Common Origin of Green Luminescence in Carbon Nanodots and Graphene Quantum Dots. ACS Nano 2014, 8, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Edison, T.N.J.I.; Aseer, K.R.; Perumal, S.; Karthik, N.; Lee, Y.R. Highly Fluorescent Nitrogen-doped Carbon Dots Derived from Phyllanthus Acidus Utilized as A Fluorescent Probe for Label-Free Selective Detection of Fe3+ Ions, Live Cell Imaging and Fluorescent Ink. Biosens. Bioelectron. 2017, 99, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.M.; Cao, F.P.; Li, Y.B. Solid Phase Synthesis of Nitrogen and Phosphor Co-Doped Carbon Quantum Dots for Sensing Fe3+ and the Enhanced Photocatalytic Degradation of Dyes. Sens. Actuators B 2017, 255, 1105–1111. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing Graphene Quantum Dots and Carbon Dots: Properties, Syntheses, and Biological Applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.X.; Xiong, S.J.; Wu, X.L.; Xu, T.; Zhu, X.B.; Gan, X.; Guo, J.H.; Shen, J.C.; Sun, L.T.; Chu, P.K. Mechanism of Photoluminescence from Chemically Derived Graphene Oxide: Role of Chemical Reduction. Adv. Opt. Mater. 2013, 1, 926–932. [Google Scholar] [CrossRef]
- Fang, X.Y.; Guo, S.J.; Li, D.; Zhu, C.Z.; Ren, W.; Dong, S.J.; Wang, E.K. Easy Synthesis and Imaging Applications of Cross-Linked Green Fluorescent Hollow Carbon Nanoparticles. ACS Nano 2012, 6, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Wu, L.; Gao, N.; Ren, J.S.; Qu, X.G. Improvement of Photoluminescence of Graphene Quantum Dots with a Biocompatible Photochemical Reduction Pathway and Its Bioimaging Application. ACS Appl. Mater. Inter. 2013, 5, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.H.; Kim, D.H.; Jun, G.H.; Hong, S.H.; Jeon, S. Tuning the Photoluminescence of Graphene Quantum Dots through the Charge Transfer Effect of Functional Groups. ACS Nano 2013, 7, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Cushing, S.K.; Li, M.; Huang, F.Q.; Wu, N.Q. Origin of Strong Excitation Wavelength Dependent Fluorescence of Graphene Oxide. ACS Nano 2014, 8, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Kozak, O.; Datta, K.K.R.; Greplova, M.; Ranc, V.; Kaslik, J.; Zboril, R. Surfactant-Derived Amphiphilic Carbon Dots with Tunable Photoluminescence. J. Phys. Chem. C 2013, 117, 24991–24996. [Google Scholar] [CrossRef]
- Dong, Y.Q.; Shao, J.W.; Chen, C.Q.; Li, H.; Wang, R.X.; Chi, Y.W.; Lin, X.M.; Chen, G.N. Blue Luminescent Graphene Quantum Dots and Graphene Oxide Prepared by Tuning the Carbonization Degree of Citric Acid. Carbon 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Qu, S.N.; Wang, X.Y.; Lu, Q.P.; Liu, X.Y.; Wang, L.J. A Biocompatible Fluorescent Ink Based on Water-Soluble Luminescent Carbon Nanodots. Angew. Chem. Int. Ed. 2012, 51, 12215–12218. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Hai, X.; Mao, Q.X.; Chen, M.L; Wang, J.H. Polyhedral Oligomeric Silsesquioxane Functionalized Carbon Dots for Cell Imaging. ACS Appl. Mater. Inter. 2015, 7, 16609–16616. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Li, M.J.; Li, Q.S.; Liang, S.J.; Tan, Y.Y.; Sheng, L.; Shi, W.; Zhang, S.X.A. Carbon Dots with Continuously Tunable Full-Color Emission and Their Application in Ratiometric PH Sensing. Chem. Mater. 2014, 26, 3104–3112. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhang, Y.; Wang, Y.; Kalytchuk, S.; Kershaw, S.V.; Wang, Y.H.; Wang, P.; Zhang, T.Q.; Zhao, Y.; Zhang, H.Z.; et al. Color-Switchable Electroluminescence of Carbon Dot Light-Emitting Diodes. ACS Nano 2013, 7, 11234–11241. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Jang, M.H.; Ha, H.D.; Kim, J.H.; Cho, Y.H.; Seo, T.S. Facile Synthetic Method for Pristine Graphene Quantum Dots and Graphene Oxide Quantum Dots: Origin of Blue and Green Luminescence. Adv. Mater. 2013, 25, 3657–3662. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Liu, Y.Y.; Yeom, S.; Kim, D.Y.; Richards, C.I. Single-Particle Fluorescence Intensity Fluctuations of Carbon Nanodots. Nano Lett. 2014, 14, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, W.; Zhou, T.Y.; Wang, B.; Li, H.Y.; Ding, L. Synthesis and Formation Mechanistic Investigation of Nitrogen-Doped Carbon Dots with High Quantum Yields and Yellowish-Green Fluorescence. Nanoscale 2016, 8, 11185–11193. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.J.; Zhang, J.H.; Tang, S.J.; Qiao, C.Y.; Wang, L.; Wang, H.Y.; Liu, X.; Li, B.; Li, Y.F.; Yu, W.L.; et al. Surface Chemistry Routes to Modulate the Photoluminescence of Graphene Quantum Dots: From Fluorescence Mechanism to Up-Conversion Bioimaging Applications. Adv. Funct. Mater. 2012, 22, 4732–4740. [Google Scholar] [CrossRef]
- Li, H.; Sun, C.H.; Ali, M.; Zhou, F.L.; Zhang, X.Y.; MacFarlane, D.R. Sulfated Carbon Quantum Dots as Efficient Visible-light Switchable Acid Catalysts for Room-Temperature Ring-Opening Reactions. Angew. Chem. Int. Ed. 2015, 54, 8420–8424. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Yan, L.H.; Si, J.H.; Hou, X. Femtosecond Laser-Assisted Synthesis of Highly Photoluminescent Carbon Nanodots for Fe3+ Detection with High Sensitivity and Selectivity. Carbon 2016, 6, 312–320. [Google Scholar]
- Zhu, S.J.; Shao, J.; Song, Y.; Zhao, X.H.; Du, J.L.; Wang, L.; Wang, H.Y.; Zhang, K.; Zhang, J.H.; Yang, B. Deeply Investigating the Surface State of Graphene Guantum Dots. Nanoscale 2015, 7, 7927–7933. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Zhang, Z.L.; Tian, Z.Q.; Zhang, L.; Liu, C.; Lin, Y.; Qi, B.; Pang, D.W. Electrochemical Tuning of Luminescent Carbon nanodots: From Preparation to Luminescence Mechanism. Adv. Mater. 2011, 23, 5801–5806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, J.H. Facile Synthesis of S, N Co-Doped Carbon Dots and Investigation of Their Photoluminescence Properties. Phys. Chem. Chem. Phys. 2015, 17, 20154–20159. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Sun, Z.C.; Zheng, M.; Li, J.; Zhang, Y.Q.; Zhang, G.Q.; Zhao, H.F.; Liu, X.Y.; Xie, Z.G. Three Colors Emission from S, N Co-Doped Graphene Quantum Dots for Visible Light H2 Production and Bioimaging. Adv. Opt. Mater. 2015, 3, 360–367. [Google Scholar] [CrossRef]
- Qu, D.; Zheng, M.; Zhang, L.G.; Zhao, H.F.; Xie, Z.G.; Jing, X.B.; Haddad, R.E.; Fan, H.Y.; Sun, Z.C. Formation Mechanism and Optimization of Highly Luminescent N-Doped Graphene Quantum Dots. Sci. Rep. 2015, 4, 5294. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zou, D.Q.; Xie, Z.; Zhang, G.P.; Li, Z.L.; Wang, C.K. Protonation Deprotonation Effects on Charge Transports of Butane-Based Molecular Junctions. Chem. Phys. Lett. 2013, 588, 155–159. [Google Scholar] [CrossRef]
- Hsu, P.C.; Chang, H.T. Synthesis of High-quality Carbon Nano Dots from Hydrophilic Compounds: Role of Functional Groups. Chem. Commun. 2012, 48, 3984–3986. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Zou, H.Y.; Yang, T.; Liu, Z.X.; Liu, H.; Huang, C.Z. An Inner Filter Effect Based Sensor of Tetracycline Hydrochloride as Developed by Loading Photoluminescent Carbon Nanodots in the Electrospun Nanofibers. Nanoscale 2016, 8, 2999–3007. [Google Scholar] [CrossRef] [PubMed]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid Functionals Based on A Screened Coulomb Potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Liu, C.; Yuan, K.; Lu, Z.; Cheng, Y.; Li, L.; Zhang, X.; Jin, P.; Meng, F.; Liu, H. Luminescence Mechanism of Carbon Dots by Tailoring Functional Groups for Sensing Fe3+ Ions. Nanomaterials 2018, 8, 233. https://doi.org/10.3390/nano8040233
Yu J, Liu C, Yuan K, Lu Z, Cheng Y, Li L, Zhang X, Jin P, Meng F, Liu H. Luminescence Mechanism of Carbon Dots by Tailoring Functional Groups for Sensing Fe3+ Ions. Nanomaterials. 2018; 8(4):233. https://doi.org/10.3390/nano8040233
Chicago/Turabian StyleYu, Jingjing, Chang Liu, Kang Yuan, Zunming Lu, Yahui Cheng, Lanlan Li, Xinghua Zhang, Peng Jin, Fanbin Meng, and Hui Liu. 2018. "Luminescence Mechanism of Carbon Dots by Tailoring Functional Groups for Sensing Fe3+ Ions" Nanomaterials 8, no. 4: 233. https://doi.org/10.3390/nano8040233
APA StyleYu, J., Liu, C., Yuan, K., Lu, Z., Cheng, Y., Li, L., Zhang, X., Jin, P., Meng, F., & Liu, H. (2018). Luminescence Mechanism of Carbon Dots by Tailoring Functional Groups for Sensing Fe3+ Ions. Nanomaterials, 8(4), 233. https://doi.org/10.3390/nano8040233