Effect of Organic Substrates on the Photocatalytic Reduction of Cr(VI) by Porous Hollow Ga2O3 Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Porous Hollow Ga2O3 Nanoparticles
2.3. Characterization
2.4. Photocatalytic Experiments
3. Results and Discussion
3.1. Composition and Morphology
3.2. Hermogravimetry and Differential Scanning Calorimetry TG-DSC Analysis
3.3. Photocatalytic Experiments
3.3.1. Photocatalytic Reduction of Cr(VI)
3.3.2. Photocatalytic Degradation of Organic Pollutants
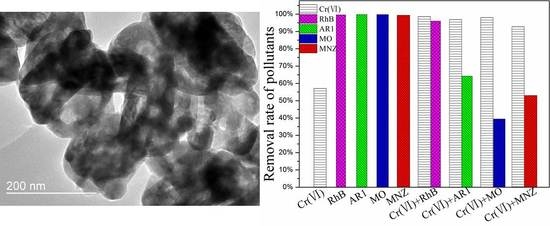
3.3.3. Simultaneous Treatment of Cr(VI) and Organic Pollutants
3.3.4. Effect of Substrate Concentration on Photocatalytic Reduction of Cr(VI)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiang, W.J.; Cai, Q.; Xu, W.; Yang, M.W.; Cai, Y.; Dionysiou, D.D.; O’Shea, K.E. Cr(VI) adsorption and reduction by humic acid coated on magnetite. Environ. Sci. Technol. 2014, 48, 8078–8085. [Google Scholar] [CrossRef] [PubMed]
- Naimi-Joubani, M.; Shirzad-Siboni, M.; Yang, J.-K.; Gholami, M.; Farzadkia, M. Photocatalytic reduction of hexavalent chromium with illuminated ZnO/TiO2 composite. J. Ind. Eng. Chem. 2015, 22, 317–323. [Google Scholar] [CrossRef]
- Yuan, X.; Zhou, C.; Jing, Q.; Tang, Q.; Mu, Y.; Du, A.-K. Facile Synthesis of g-C3N4 Nanosheets/ZnO Nanocomposites with Enhanced Photocatalytic Activity in Reduction of Aqueous Chromium(VI) under Visible Light. Nanomaterials 2016, 6, 173. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Qin, F.; Ming, Y.A.; Zhao, H.P.; Liu, Y.L.; Chen, R. Fabrication uniform hollow Bi2S3 nanospheres via Kirkendall effect for photocatalytic reduction of Cr(VI) in electroplating industry wastewater. J. Hazard. Mater. 2017, 340, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Abdel Moniem, S.M.; Ali, M.E.M.; Gad-Allah, T.A.; Khalil, A.S.G.; Ulbricht, M.; El-Shahat, M.F.; Ashmawy, A.M.; Ibrahim, H.S. Detoxification of hexavalent chromium in wastewater containing organic substances using simonkolleite-TiO2 photocatalyst. Process Saf. Environ. Prot. 2015, 95, 247–254. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Li, J.; Zhang, M.; Dionysiou, D.D. Size-tunable hydrothermal synthesis of SnS2 nanocrystals with high performance in visible light-driven photocatalytic reduction of aqueous Cr(VI). Environ. Sci. Technol. 2011, 45, 9324–9331. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Zhou, X.S.; Zhang, L.L.; Xu, L.M.; Ma, L.; Luo, J.; Li, M.J.; Zeng, L.H. Photoredox degradation of different water pollutants (MO, RhB, MB, and Cr(VI)) using Fe-N-S-tri-doped TiO2 nanophotocatalyst prepared by novel chemical method. Mater. Res. Bull. 2015, 70, 106–113. [Google Scholar] [CrossRef]
- Meng, X.D.; Zhang, G.K.; Li, N. Bi24Ga2O39 for visible light photocatalytic reduction of Cr(VI): Controlled synthesis, facet-dependent activity and DFT study. Chem. Eng. J. 2017, 314, 249–256. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, G.K.; Wu, X.Y.; Yin, S. Novel visible-light-driven Z-scheme Bi12GeO20/g-C3N4 photocatalyst: Oxygen-induced pathway of organic pollutants degradation and proton assisted electron transfer mechanism of Cr(VI) reduction. Appl. Catal. B 2017, 207, 17–26. [Google Scholar] [CrossRef]
- Wang, P.; Ji, W.D.; Li, M.M.; Zhang, G.K.; Wang, J.L. Bi25VO40 microcube with step surface for visible light photocatalytic reduction of Cr(VI): Enhanced activity and ultrasound assisted regeneration. Ultrason. Sonochem. 2017, 38, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Vera, M.L.; Traid, H.D.; Henrikson, E.R.; Ares, A.E.; Litter, M.I. Heterogeneous photocatalytic Cr(VI) reduction with short and long nanotubular TiO2 coatings prepared by anodic oxidation. Mater. Res. Bull. 2018, 97, 150–157. [Google Scholar] [CrossRef]
- Sun, B.; Reddy, E.P.; Smirniotis, P.G. Visible light Cr(VI) reduction and organic chemical oxidation by TiO2 photocatalysis. Environ. Sci. Technol. 2005, 39, 6251–6259. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.H.; Chan, Q.Z.; Wu, X.J.; Wang, H.J.; Liu, Y.L. The simultaneous photocatalytic degradation of phenol and reduction of Cr(VI) by TiO2/CNTs. J. Ind. Eng. Chem. 2012, 18, 574–580. [Google Scholar] [CrossRef]
- Liu, F.H.; Yu, J.; Tu, G.Y.; Qu, L.; Xiao, J.C.; Liu, Y.D.; Wang, L.Z.; Lei, J.Y.; Zhang, J.L. Carbon nitride coupled Ti-SBA15 catalyst for visible-light-driven photocatalytic reduction of Cr(VI) and the synergistic oxidation of phenol. Appl. Catal. B 2017, 201, 1–11. [Google Scholar] [CrossRef]
- Li, X.F.; Zhen, X.Z.; Meng, S.G.; Xian, J.J.; Shao, Y.; Fu, X.Z.; Li, D.Z. Structuring β-Ga2O3 photonic crystal photocatalyst for efficient degradation of organic pollutants. Environ. Sci. Technol. 2013, 47, 9911–9917. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Zhang, P.Y.; Jin, L.; Li, Z.M. Photocatalytic decomposition of perfluorooctanoic acid in pure water and sewage water by nanostructured gallium oxide. Appl. Catal. B 2013, 142–143, 654–661. [Google Scholar] [CrossRef]
- Girija, K.; Thirumalairajan, S.; Mastelaro, V.R.; Mangalaraj, D. Photocatalytic degradation of organic pollutants by shape selective synthesis of β-Ga2O3 microspheres constituted by nanospheres for environmental remediation. J. Mater. Chem. A 2015, 3, 2617–2627. [Google Scholar] [CrossRef]
- Pan, Y.X.; Sun, Z.Q.; Cong, H.P.; Men, Y.L.; Xin, S.; Song, J.; Yu, S.H. Photocatalytic CO2 reduction highly enhanced by oxygen vacancies on Pt-nanoparticle-dispersed gallium oxide. Nano Res. 2016, 9, 1689–1700. [Google Scholar] [CrossRef]
- Chen, S.L.; Li, D.; Liu, Y.X.; Huang, W.X. Morphology-dependent defect structures and photocatalytic performance of hydrogenated anatase TiO2 nanocrystals. J. Catal. 2016, 341, 126–135. [Google Scholar] [CrossRef]
- Liu, J.; Lu, W.; Tian, B.S.; Hu, B.; Jin, L.; Shi, Y.R.; Li, L.; Wang, Z.L. Shape-controlled synthesis and facet-dependent performance of single-crystal Bi25GaO39 photocatalysts. CrystEngComm 2016, 18, 7715–7721. [Google Scholar] [CrossRef]
- Liu, J.; Lu, W.; Zhong, Q.; Jin, X.D.; Wei, L.Y.; Wu, H.Z.; Zhang, X.L.; Li, L.L.; Wang, Z.L. Single-crystal Bi2Ga4O9 nanoplates with exposed {110} facets for photocatalytic degradation of Acid Red 1. Mol. Catal. 2017, 433, 354–362. [Google Scholar] [CrossRef]
- Parlett, C.M.A.; Wilson, K.; Lee, A.F. Hierarchical porous materials: Catalytic applications. Chem. Soc. Rev. 2013, 42, 3876–3893. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.C.; Tang, L.; Zeng, G.M.; Zhu, Z.J.; Yan, M.; Zhou, Y.Y.; Wang, J.J.; Liu, Y.N.; Wang, J.J. Insight into highly efficient simultaneous photocatalytic removal of Cr(VI) and 2,4-diclorophenol under visible light irradiation by phosphorus doped porous ultrathin g-C3N4 nanosheets from aqueous media: Performance and reaction mechanism. Appl. Catal. B 2017, 203, 343–354. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, J.; Zong, R.L.; Zhu, Y.F. Enhancement of visible light photocatalytic activities via porous structure of g-C3N4. Appl. Catal. B 2014, 147, 229–235. [Google Scholar] [CrossRef]
- Liu, J.; Lu, W.; Zhong, Q.; Wu, H.Z.; Li, Y.L.; Li, L.L.; Wang, Z.L. Effect of pH on the microstructure of β-Ga2O3 and its enhanced photocatalytic activity for antibiotic degradation. J. Colloid Interface Sci. 2018, 519, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, W.; Wu, H.Z.; Jin, L.; Hu, B.; Li, L.L.; Wang, Z.L. In situ synthesis of rice-like ZnGa2O4 for the photocatalytic removal of organic and inorganic pollutants. Mater. Sci. Semicond. Process. 2016, 56, 251–259. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Q.; Li, M.R.; Shen, S.; Wang, X.L.; Wang, Y.C.; Feng, Z.C.; Shi, J.Y.; Han, H.X.; Li, C. Photocatalytic overall water splitting promoted by an α-β phase junction on Ga2O3. Angew. Chem. Int. Ed. 2012, 51, 13089–13092. [Google Scholar] [CrossRef] [PubMed]
- Machon, D.; McMillan, P.F.; Xu, B.; Dong, J.J. High-pressure study of the β-to-α transition in Ga2O3. Phys. Rev. B 2006, 73, 094125. [Google Scholar] [CrossRef]
- Hou, Y.D.; Wu, L.; Wang, X.C.; Ding, Z.X.; Li, Z.H.; Fu, X.Z. Photocatalytic performance of α-, β-, and γ-Ga2O3 for the destruction of volatile aromatic pollutants in air. J. Catal. 2007, 250, 12–18. [Google Scholar] [CrossRef]
- Guo, S.; Yuan, N.; Zhang, G.K.; Yu, J.C. Graphene modified iron sludge derived from homogeneous Fenton process as an efficient heterogeneous Fenton catalyst for degradation of organic pollutants. Microporous Mesoporous Mater. 2017, 238, 62–68. [Google Scholar] [CrossRef]
- Girija, K.; Thirumalairajan, S.; Patra, A.K.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C. Enhanced photocatalytic performance of novel self-assembled floral β-Ga2O3 nanorods. Curr. Appl. Phys. 2013, 13, 652–658. [Google Scholar] [CrossRef]
- Barrera-Díaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater. 2012, 223–224, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alidokht, L.; Khataee, A.R.; Reyhanitabar, A.; Oustan, S. Reductive removal of Cr(VI) by starch-stabilized Fe0 nanoparticles in aqueous solution. Desalination 2011, 270, 105–110. [Google Scholar] [CrossRef]
- Liang, R.W.; Shen, L.J.; Jing, F.F.; Wu, W.M.; Qin, N.; Lin, R.; Wu, L. NH2-mediated indium metal-organic framework as a novel visible-light-driven photocatalyst for reduction of the aqueous Cr(VI). Appl. Catal. B 2015, 162, 245–251. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.Z.; Wu, Y.; Zeng, G.M.; Chen, X.H.; Leng, L.J.; Wu, Z.B.; Jiang, L.B.; Li, H. Facile synthesis of amino-functionalized titanium metal-organic frameworks and their superior visible-light photocatalytic activity for Cr(VI) reduction. J. Hazard. Mater. 2015, 286, 187–194. [Google Scholar] [CrossRef] [PubMed]










| Name | Single Pollutant System, mg/L | Binary Pollutant System, mg/L | |||
|---|---|---|---|---|---|
| RhB + Cr(VI) | AR1 + Cr(VI) | MO + Cr(VI) | MNZ + Cr(VI) | ||
| Cr(VI) | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| RhB | 5 | 5 | × | × | × |
| AR1 | 20 | × | 20 | × | × |
| MO | 10 | × | × | 10 | × |
| MNZ | 20 | × | × | × | 20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Gan, H.; Wu, H.; Zhang, X.; Zhang, J.; Li, L.; Wang, Z. Effect of Organic Substrates on the Photocatalytic Reduction of Cr(VI) by Porous Hollow Ga2O3 Nanoparticles. Nanomaterials 2018, 8, 263. https://doi.org/10.3390/nano8040263
Liu J, Gan H, Wu H, Zhang X, Zhang J, Li L, Wang Z. Effect of Organic Substrates on the Photocatalytic Reduction of Cr(VI) by Porous Hollow Ga2O3 Nanoparticles. Nanomaterials. 2018; 8(4):263. https://doi.org/10.3390/nano8040263
Chicago/Turabian StyleLiu, Jin, Huihui Gan, Hongzhang Wu, Xinlei Zhang, Jun Zhang, Lili Li, and Zhenling Wang. 2018. "Effect of Organic Substrates on the Photocatalytic Reduction of Cr(VI) by Porous Hollow Ga2O3 Nanoparticles" Nanomaterials 8, no. 4: 263. https://doi.org/10.3390/nano8040263
APA StyleLiu, J., Gan, H., Wu, H., Zhang, X., Zhang, J., Li, L., & Wang, Z. (2018). Effect of Organic Substrates on the Photocatalytic Reduction of Cr(VI) by Porous Hollow Ga2O3 Nanoparticles. Nanomaterials, 8(4), 263. https://doi.org/10.3390/nano8040263