Transition Metal Hollow Nanocages as Promising Cathodes for the Long-Term Cyclability of Li–O2 Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Mn3O4 Hollow Nanocages (MOHNs)
2.3. Physicochemical Characterizations of MOHNs
2.4. Preparation of MOHNs/KB Cathode and Its Li–O2 Battery
2.5. Electrochemical Characterizations of Li–O2 Battery
3. Results and Discussion
3.1. Physicochemical Properties of MOHNs
3.2. Proposed Formation Mechanism of MOHNs
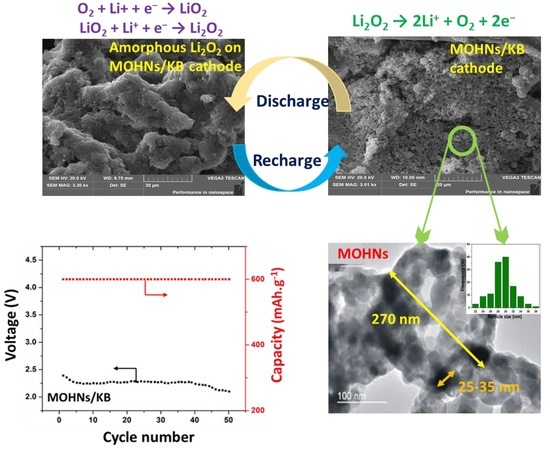
3.3. Electrochemical Performance of Li–O2 Battery
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Larcher, D.; Tarascon, J.-M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2014, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 2011, 11, 172. [Google Scholar] [CrossRef]
- Eftekhari, A.; Ramanujam, B. In pursuit of catalytic cathodes for lithium–oxygen batteries. J. Mater. Chem. A 2017, 5, 7710–7731. [Google Scholar] [CrossRef]
- Shao, Y.; Park, S.; Xiao, J.; Zhang, J.G.; Wang, Y.; Liu, J. Electrocatalysts for nonaqueous lithium-air batteries: Status, challenges, and perspective. ACS Catal. 2012, 2, 844–857. [Google Scholar] [CrossRef]
- Beattie, S.D.; Manolescu, D.M.; Blair, S.L. High-capacity lithium–air cathodes. J. Electrochem. Soc. 2009, 156, A44–A47. [Google Scholar] [CrossRef]
- Imanishi, N.; Yamamoto, O. Rechargeable lithium-air batteries: Characteristics and prospects. Mater. Today 2014, 17, 24–30. [Google Scholar] [CrossRef]
- Guo, X.; Sun, B.; Su, D.; Liu, X.; Liu, H.; Wang, Y.; Wang, G. Recent developments of aprotic lithium-oxygen batteries: Functional materials determine the electrochemical performance. Sci. Bull. 2017, 62, 442–452. [Google Scholar] [CrossRef]
- Ma, Z.; Shao, G.; Fan, Y.; Wang, G.; Song, J.; Shen, D. Construction of hierarchical α-MnO2 nanowires@ultrathin δ-MnO2 nanosheets core-shell nanostructure with excellent cycling stability for high-power asymmetric supercapacitor electrodes. ACS Appl. Mater. Interfaces 2016, 8, 9050–9058. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-S.; Kim, J.-H.; Kim, K.J.; Jeong, G.; Kim, Y.-J. Morphological modification of α-MnO2 catalyst for use in Li/air batteries. J. Nanosci. Nanotechnol. 2013, 13, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- Débart, A.; Paterson, A.J.; Bao, J.; Bruce, P.G. α-MnO2 nanowires: A catalyst for the O2 electrode in rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008, 47, 4521–4524. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Sun, Y.; Liu, L.; Xu, S.; Yan, X. Advances in manganese-based oxides cathodic electrocatalysts for Li-air batteries. Adv. Funct. Mater. 2018, 28, 1704973. [Google Scholar] [CrossRef]
- Park, M.-S.; Kim, J.; Kim, K.J.; Lee, J.-W.; Kim, J.H.; Yamauchi, Y. Porous nanoarchitectures of spinel-type transition metal oxides for electrochemical energy storage systems. Phys. Chem. Chem. Phys. 2015, 17, 30963–30977. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Lal, S. Orbital and spin ordering physics of the Mn3O4 spinel. Phys. Rev. B 2017, 96, 1–10. [Google Scholar] [CrossRef]
- Lv, K.; Zhang, Y.; Zhang, D.; Ren, W.; Sun, L. Mn3O4 nanoparticles embedded in 3D reduced graphene oxide network as anode for high-performance lithium ion batteries. J. Mater. Sci. Mater. Electron. 2017, 28, 14919–14927. [Google Scholar] [CrossRef]
- Ramírez, A.; Hillebrand, P.; Stellmach, D.; May, M.M.; Bogdanoff, P.; Fiechter, S. Evaluation of MnOx, Mn2O3, and Mn3O4 electrodeposited films for the oxygen evolution reaction of water. J. Phys. Chem. C 2014, 118, 14073–14081. [Google Scholar] [CrossRef]
- Cao, Y.; Or, S.W. Enhanced cyclability in rechargeable Li–O2 batteries based on Mn3O4 hollow nanocage/Ketjenblack catalytic air cathode. IEEE Trans. Mag. 2016, 52, 9100504. [Google Scholar] [CrossRef]
- Li, Q.; Xu, P.; Zhang, B.; Tsai, H.; Wang, J.; Wang, H.-L.; Wu, G. One-step synthesis of Mn3O4/reduced graphene oxide nanocomposites for oxygen reduction in nonaqueous Li–O2 batteries. Chem. Commun. 2013, 49, 10838–10840. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-N.; Lee, J.-I.; Yoon, S.; Yeon, S.-H.; Chang, W.; Shin, K.-H.; Lee, J.-W. Manganese oxide/carbon composite nanofibers: Electrospinning preparation and application as a bi-functional cathode for rechargeable lithium–oxygen batteries. J. Mater. Chem. 2012, 22, 21845–21848. [Google Scholar] [CrossRef]
- Abdelaal, H.M. Template-assisted synthesis of metal oxide hollow spheres utilizing glucose derived-carbonaceous spheres as sacrificial templates. J. Adv. Chem. Eng. 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Yue, J.; Gu, X.; Chen, L.; Wang, N.; Jiang, X.; Xu, H.; Yang, J.; Qian, Y. General synthesis of hollow MnO2, Mn3O4 and MnO nanospheres as superior anode materials for lithium ion batteries. J. Mater. Chem. A 2014, 2, 17421–17426. [Google Scholar] [CrossRef]
- Lei, S.; Tang, K.; Fang, Z.; Zheng, H. Ultrasonic-assisted synthesis of colloidal Mn3O4 nanoparticles at normal temperature and pressure. Cryst. Growth Des. 2006, 6, 1757–1760. [Google Scholar] [CrossRef]
- Yang, L.X.; Liang, Y.; Chen, H.; Meng, Y.F.; Jiang, W. Controlled synthesis of Mn3O4and MnCO3 in a solvothermal system. Mater. Res. Bull. 2009, 44, 1753–1759. [Google Scholar] [CrossRef]
- Toufiq, A.M.; Wang, F.; Javed, U.-Q.-A.; Li, Q.; Li, Y.; Khan, M. Synthesis, characterization and photoluminescent properties of 3D nanostructures self-assembled with Mn3O4 nanoparticles. Mater. Express 2014, 4, 258–262. [Google Scholar] [CrossRef]
- Zuo, J.; Xu, C.; Liu, Y.; Qian, Y. Crystallite size effects on the Raman spectra of Mn3O4. Nanostruct. Mater. 1998, 10, 1331–1335. [Google Scholar] [CrossRef]
- Guo, H.; Li, T.; Chen, W.; Liu, L.; Qiao, J.; Zhang, J. Self-assembly formation of hollow Ni-Fe-O nanocage architectures by metal-organic frameworks with high-performance lithium storage. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Gupta, D.; Patra, A.K.; Saha, B.; Bhaumik, A. Synthesis of 5-hydroxymethylfurural from carbohydrates using large-pore mesoporous tin phosphate. ChemSusChem 2014, 7, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Dhaouadi, H.; Ghodbane, O.; Hosni, F.; Touati, F. Nanoparticles: Synthesis, characterization, and dielectric Properties. ISRN Spectrosc. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Fang, M.; Tan, X.; Liu, M.; Kang, S.; Hu, X.; Zhang, L. Low-temperature synthesis of Mn3O4 hollow-tetrakaidecahedrons and their application in electrochemical capacitors. CrystEngComm 2011, 13, 4915–4920. [Google Scholar] [CrossRef]
- Ahn, S.M.; Suk, J.; Kim, D.Y.; Kang, Y.; Kim, H.K.; Kim, D.W. High-performance lithium-oxygen battery electrolyte derived from optimum combination of solvent and lithium salt. Adv. Sci. 2017, 4, 1700235. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Kim, H.J.; Back, S.; Nam, K.W.; Jung, Y.; Han, Y.K.; Choi, J.W. Improved reversibility in lithium-oxygen battery: Understanding elementary reactions and surface charge engineering of metal alloy catalyst. Sci. Rep. 2015, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Fenske, D.; Parisi, J. Study on electrolyte stability and oxygen reduction reaction mechanisms in the presence of manganese oxide catalysts for aprotic lithium–oxygen batteries. Energy Technol. 2016, 4, 1531–1542. [Google Scholar] [CrossRef]
- Younesi, R.; Hahlin, M.; Roberts, M.; Edström, K. The SEI layer formed on lithium metal in the presence of oxygen: A seldom considered component in the development of the Li–O2 battery. J. Power Sources 2013, 225, 40–45. [Google Scholar] [CrossRef]
- Assary, R.S.; Lu, J.; Du, P.; Luo, X.; Zhang, X.; Ren, Y. The effect of oxygen crossover on the anode of a Li–O2 battery using an ether-based solvent: Insights from experimental and computational studies. ChemSusChem 2013, 6, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Kichambare, P.; Kumar, J.; Rodrigues, S.; Kumar, B. Electrochemical performance of highly mesoporous nitrogen doped carbon cathode in lithium–oxygen batteries. J. Power Sources 2011, 196, 3310–3316. [Google Scholar] [CrossRef]
- Mirzaeian, M.; Hall, P.J. Characterizing capacity loss of lithium oxygen batteries by impedance spectroscopy. J. Power Sources 2010, 195, 6817–6824. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Huang, Z.; Oh, S.; Yu, Y.; Mai, Y.-W.; Kim, J.-K. Urchin-like Li4Ti5O12–carbon nanofiber composites for high rate performance anodes in Li-ion batteries. J. Mater. Chem. 2012, 22, 12133–12140. [Google Scholar] [CrossRef]
- Landa-Medrano, I.; de Larramendi, I.R.; Ortiz-Vitoriano, N.; Pinedo, R.; de Larramendi, J.I.; Rojo, T. In situ monitoring of discharge/charge processes in Li–O2 batteries by electrochemical impedance spectroscopy. J. Power Sources 2014, 249, 110–117. [Google Scholar] [CrossRef]
- Read, J. Characterization of the lithium/oxygen organic electrolyte battery. J. Electrochem. Soc. 2002, 149, A1190–A1195. [Google Scholar] [CrossRef]
- Wang, F.; Xu, Y.H.; Luo, Z.K.; Pang, Y.; Wu, Q.X.; Liang, C.S.; Chen, J.; Liu, D.; Zhang, X.H. A dual pore carbon aerogel based air cathode for a highly rechargeable lithium-air battery. J. Power Sources 2014, 272, 1061–1071. [Google Scholar] [CrossRef] [Green Version]
- Adams, B.D.; Radtke, C.; Black, R.; Trudeau, M.L.; Zaghib, K.; Nazar, L.F. Current density dependence of peroxide formation in the Li–O2 battery and its effect on charge. Energy Environ. Sci. 2013, 6, 1772–1778. [Google Scholar] [CrossRef]
- Kwak, W.-J.; Lau, K.C.; Shin, C.-D.; Amine, K.; Curtiss, L.A.; Sun, Y.-K. A Mo2C/Carbon nanotube composite cathode for lithium–oxygen batteries with high energy efficiency and long cycle life. ACS Nano 2015, 9, 4129–4137. [Google Scholar] [CrossRef] [PubMed]
- Kwak, K.-H.; Kim, D.W.; Kang, Y.; Suk, J. Hierarchical Ru- and RuO2-foams as high performance electrocatalysts for rechargeable lithium–oxygen batteries. J. Mater. Chem. A 2016, 4, 16356–16367. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Gallant, B.M.; Kwabi, D.G.; Harding, J.R.; Mitchell, R.R.; Whittingham, M.S.; Shao-Horn, Y. Lithium–oxygen batteries: Bridging mechanistic understanding and battery performance. Energy Environ. Sci. 2013, 6, 750–768. [Google Scholar] [CrossRef]
- Yin, W.; Shen, Y.; Zou, F.; Hu, X.; Chi, B.; Huang, Y. Metal-organic framework derived ZnO/ZnFe2O4/C nanocages as stable cathode material for reversible lithium-oxygen batteries. ACS Appl. Mater. Interfaces 2015, 7, 4947–4954. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhang, S.S.; Tran, D.T.; Read, J. Oxygen reduction reaction catalyst on lithium/air battery discharge performance. J. Mater. Chem. 2011, 21, 10118–10125. [Google Scholar] [CrossRef]
- Mohamed, S.G.; Tsai, Y.-Q.; Chen, C.-J.; Tsai, Y.-T.; Hung, T.-F.; Chang, W.-S.; Liu, R.-S. Ternary spinel MCo2O4 (M = Mn, Fe, Ni, and Zn) porous nanorods as bifunctional cathode materials for lithium–O2 batteries. ACS Appl. Mater. Interfaces 2015, 7, 12038–12046. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-C.; Gasteiger, H.A.; Parent, M.C.; Chiloyan, V.; Shao-Horn, Y. The influence of catalysts on discharge and charge voltages of rechargeable Li–oxygen batteries. Electrochem. Solid-State Lett. 2010, 13, A69–A72. [Google Scholar] [CrossRef]
- Tan, P.; Wei, Z.H.; Shyy, W.; Zhao, T.S.; Zhu, X.B. A nano-structured RuO2/NiO cathode enables the operation of non-aqueous lithium–air batteries in ambient air. Energy Environ. Sci. 2016, 9, 1783–1793. [Google Scholar] [CrossRef]
- Chen, Y.M.; Xie, A.; Zhu, Y. Cyclability of a lithium-oxygen battery containing N-Methyl-2-Pyrrolidone and a vertically aligned carbon nanotube cathode. ChemElectroChem 2015, 2, 208–212. [Google Scholar] [CrossRef]
- Mette, K.; Bergmann, A.; Tessonnier, J.-P.; Hävecker, M.; Yao, L.; Ressler, T.; Schlögl, R.; Strasser, P.; Behrens, M. Nanostructured manganese oxide supported on carbon nanotubes for electrocatalytic water splitting. ChemCatChem 2012, 4, 851–862. [Google Scholar] [CrossRef]
- Risch, M.; Stoerzinger, K.A.; Han, B.; Regier, T.Z.; Peak, D.; Sayed, S.Y.; Wei, C.; Xu, Z.; Shao-Horn, Y. Redox processes of manganese oxide in catalyzing oxygen evolution and reduction: An in situ soft X-ray absorption spectroscopy study. J. Phys. Chem. C 2017, 121, 17682–17692. [Google Scholar] [CrossRef]
- Recham, N.; Chotard, J.N.; Dupont, L.; Delacourt, C.; Walker, W.; Armand, M.; Tarascon, J.M. A 3.6 v lithium-based fluorosulphate insertion positive electrode for lithium-ion batteries. Nat. Mater. 2010, 9, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Feng, X.; Nie, Y.; Chen, S.; Shi, F.; Xiong, K.; Ding, W.; Qi, X.; Hu, J.; Wei, Z.; et al. Insight into the effect of oxygen vacancy concentration on the catalytic performance of MnO2. ACS Catal. 2015, 4825–4832. [Google Scholar] [CrossRef]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatterjee, A.; Or, S.W.; Cao, Y. Transition Metal Hollow Nanocages as Promising Cathodes for the Long-Term Cyclability of Li–O2 Batteries. Nanomaterials 2018, 8, 308. https://doi.org/10.3390/nano8050308
Chatterjee A, Or SW, Cao Y. Transition Metal Hollow Nanocages as Promising Cathodes for the Long-Term Cyclability of Li–O2 Batteries. Nanomaterials. 2018; 8(5):308. https://doi.org/10.3390/nano8050308
Chicago/Turabian StyleChatterjee, Amrita, Siu Wing Or, and Yulin Cao. 2018. "Transition Metal Hollow Nanocages as Promising Cathodes for the Long-Term Cyclability of Li–O2 Batteries" Nanomaterials 8, no. 5: 308. https://doi.org/10.3390/nano8050308
APA StyleChatterjee, A., Or, S. W., & Cao, Y. (2018). Transition Metal Hollow Nanocages as Promising Cathodes for the Long-Term Cyclability of Li–O2 Batteries. Nanomaterials, 8(5), 308. https://doi.org/10.3390/nano8050308