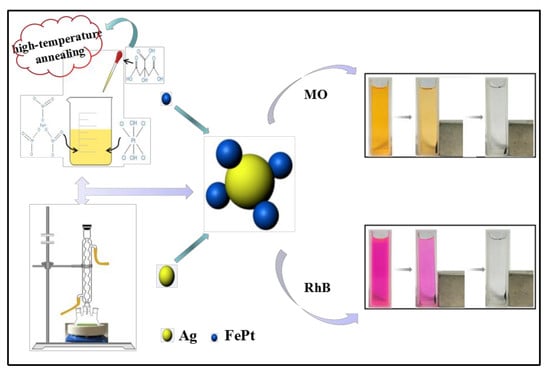
Highly Efficient, Low-Cost, and Magnetically Recoverable FePt–Ag Nanocatalysts: Towards Green Reduction of Organic Dyes
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Silver Colloids
2.3. Preparation of Ag@PEI-DTC Solution
2.4. Preparation of FePt Nanocrystals
2.5. Preparation of FePt–Ag@PEI-DTC Nanocomposites
2.6. Application of FePt–Ag Nanocomposites for Catalytic Reduction of MO and RhB
2.7. Characterization Methods
3. Results and Discussion
3.1. Structure of FePt–Ag Nanocomposites
3.2. Morphology of FePt–Ag Nanocomposites
3.3. XPS Studies of FePt–Ag Nanocomposites
3.4. Magnetism of FePt–Ag Nanocomposites
3.5. Catalytic Studies of FePt–Ag Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xie, Y.J.; Yan, B.; Xu, H.L.; Chen, J.; Liu, Q.X.; Deng, Y.H.; Zeng, H.B. Highly Regenerable Mussel-Inspired Fe3O4@Polydopamine-Ag Core-Shell Microspheres as Catalyst and Adsorbent for Methylene Blue Removal. ACS Appl. Mater. Interfaces 2014, 6, 8845–8852. [Google Scholar] [CrossRef] [PubMed]
- Deka, P.; Hazarika, A.; Dekaa, R.C.; Bharali, P. Influence of CuO morphology on the enhanced catalytic degradation of methylene blue and methyl orange. RSC Adv. 2016, 6, 95292–95306. [Google Scholar] [CrossRef]
- Xu, D.; Cheng, F.; Lu, Q.; Dai, P. Microwave Enhanced Catalytic Degradation of Methyl Orange inAqueous Solution over CuO/CeO2 Catalyst in the Absence andPresence of H2O2. Ind. Eng. Chem. Res. 2014, 53, 2625–2632. [Google Scholar] [CrossRef]
- Amir, M.D.; Kurtan, U.; Baykal, A. Rapid color degradation of organic dyes by Fe3O4@His@Ag recyclable magnetic nanocatalyst. J. Ind. Eng. Chem. 2015, 27, 347–353. [Google Scholar] [CrossRef]
- Saikia, P.; Miah, A.T.; Das, P.P. Highly efficient catalytic reductive degradation of various organic dyes by Au/CeO2-TiO2 nano-hybrid. J. Chem. Sci. 2017, 129, 81–93. [Google Scholar] [CrossRef]
- Soto-Quintero, A.; Romo-Uribe, Á.; Bermúdez-Morales, V.H.; Quijada-Garrido, I.; Guarrotxena, N. 3D-Hydrogel Based Polymeric Nanoreactors for Silver Nano-Antimicrobial Composites Generation. Nanomaterials 2017, 7, 209. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, S.M.; Almuqati, T.; Almuqati, N.; Al-Farraj, E.; Alhokbany, N.; Ahamad, T. Chitosan based polymer matrix with silver nanoparticles decorated multiwalled carbon nanotubes for catalytic reduction of 4-nitrophenol. Carbohydr. Polym. 2016, 151, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; He, Y.Y.; Sui, H.; He, L. One-Step Fabrication of Dual Responsive Lignin Coated Fe3O4 Nanoparticles for Efficient Removal of Cationic and Anionic Dyes. Nanomaterials 2018, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.M.; Abo, Z.E.F.; Elseman, A.M.; Alotaibi, N.F. A novel heterometallic compound for design and study of electrical properties of silver nanoparticles-decorated lead compounds. New J. Chem. 2018, 42, 1387–1395. [Google Scholar] [CrossRef]
- Ray, C.; Pal, T. Recent advances of metal-metal oxide nanocomposites and their tailored nanostructures in numerous catalytic applications. J. Mater Chem. A 2107, 5, 9465–9487. [Google Scholar] [CrossRef]
- Liu, Y.; Kou, Q.W.; Wang, D.D.; Chen, L.; Sun, Y.T.; Lu, Z.Y.; Zhang, Y.Y.; Wang, Y.X.; Yang, J.H.; Xing, S. Rational synthesis and tailored optical and magnetic Characteristics of Fe3O4-Au composite nanoparticles. J. Mater. Sci. 2017, 52, 10163–10174. [Google Scholar] [CrossRef]
- Gong, R.; Ye, J.J.; Dai, W.; Yan, X.Y.; Hu, J.; Hu, X.; Li, S.; Huang, H. Adsorptive Removal of Methyl Orange and Methylene Blue from Aqueous Solution with Finger-Citron-Residue-Based Activated Carbon. Ind. Eng. Chem. Res. 2013, 52, 14297–14303. [Google Scholar] [CrossRef]
- Vi, T.T.T.; Kumar, S.R.; Rout, B.; Liu, C.-H.; Wong, C.-B.; Chang, C.-W.; Chen, C.-H.; Chen, D.W.; Lue, S.J.J. The Preparation of Graphene Oxide-Silver Nanocomposites: The Effect of Silver Loads on Gram-Positive and Gram-Negative Antibacterial Activities. Nanomaterials 2018, 8, 163. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Tan, L.; Li, B.; Liu, T.; Ren, J.; Huang, Z.; Tang, F.; Meng, X. One-pot gradient solvothermal synthesis of the Ag/Au-Fe3O4 composite nanoparticles and their applications. RSC Adv. 2014, 4, 56057–56062. [Google Scholar] [CrossRef]
- Jin, C.J.; Han, J.; Chu, F.Y.; Wang, X.X.; Guo, R. Fe3O4@PANI Hybrid Shell as a Multifunctional Support for Au Nanocatalysts with a Remarkably Improved Catalytic Performance. Langmuir 2017, 33, 4520–4527. [Google Scholar] [CrossRef] [PubMed]
- Munshi, A.M.; Shi, M.; Thomas, S.P.; Saunders, M.; Spackman, M.A.; Iyer, K.S.; Smith, N.M. Magnetically recoverable Fe3O4@Au-coated nanoscale catalysts for the A3-coupling reaction. Dalton Trans. 2017, 46, 5133–5137. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Li, D.Z.; Lin, Y.M.; Wang, P.X.; Chen, W.; Fu, X.Z.; Shao, Y. Evidence for the Active Species Involved in the Photodegradation Process of Methyl Orange on TiO2. J. Phys. Chem. C 2012, 116, 3552–3560. [Google Scholar] [CrossRef]
- Xuan, S.H.; Wang, Y.-X.J.; Yu, J.C.; Leung, K.C.-F. Preparation, Characterization, and Catalytic Activity of Core/Shell Fe3O4@Polyaniline@Au Nanocomposites. Langmuir 2009, 25, 11835–11843. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gai, L.G.; Ma, W.Y.; Jiang, H.H.; Peng, X.Q.; Zhao, L.C. Ultrasound-Assisted Catalytic Degradation of Methyl Orange with Fe3O4/Polyaniline in Near Neutral Solution. Ind. Eng. Chem. Res. 2015, 54, 2279–2289. [Google Scholar] [CrossRef]
- Xiong, R.; Lu, C.; Wang, Y.; Zhou, Z.; Zhang, X. Nanofibrillated cellulose as the support and reductant for the facile synthesis of Fe3O4/Ag nanocomposites with catalytic and antibacterial activity. J. Mater. Chem. A 2013, 1, 14910–14918. [Google Scholar] [CrossRef]
- Subhan, M.A.A.; Saha, P.C.; Rahman, M.M.; Ahmed, J.; Asirib, A.M. Mohammad Al-Mamun, Fabrication of a 2,4-dinitrophenol sensor based on Fe3O4@Ag@Ni nanomaterials and studies on their antibacterial properties. New J. Chem. 2018, 42, 872–881. [Google Scholar] [CrossRef]
- Zhang, X.P.; Jiang, W.Q.; Gong, X.L.; Zhang, Z. Sonochemical synthesis and characterization of magnetic separable Fe3O4/Ag composites and its catalytic properties. J. Alloys Compd. 2010, 508, 400–405. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Y.H.; Zhang, X.L.; Wang, Y.X.; Zhang, Y.J.; Liu, H.L.; Zhai, H.J.; Liu, Y.Q.; Yang, J.H.; Yan, Y.S. Effects of annealing temperature on the structure and magnetic properties of the L10-FePt nanoparticles synthesized by the modified sol-gel method. Powder Technol. 2013, 239, 217–222. [Google Scholar] [CrossRef]
- Li, Q.; Wu, L.H.; Wu, G.; Su, D.; Lv, H.F.; Zhang, S.; Zhu, W.L.; Casimir, A.; Zhu, H.Y.; Mendoza-Garcia, A.; et al. New Approach to Fully Ordered fct-FePt Nanoparticles for Much Enhanced Electrocatalysis in Acid. Nano Lett. 2015, 15, 2468–2474. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.; Lin, P.Y. FePt nanoparticles as heterogeneous Fenton-like catalysts for hydrogen peroxide decomposition and the decolorization of methylene blue. J. Nanopart. Res. 2012, 14, 1–10. [Google Scholar] [CrossRef]
- Wang, S.Q.; Xu, L.-P.; Zhang, X.J. Ultrasensitive Electrochemical Biosensor Based on Noble Metal Nanomaterials. Sci. Adv. Mater. 2015, 7, 2084–2102. [Google Scholar] [CrossRef]
- Svedendahl, M.; Verre, R.; Kall, M. Refractometric biosensing based on optical phase flips in sparse and short-range-ordered nanoplasmonic layers. Light Sci. Appl. 2014, 3, e220. [Google Scholar] [CrossRef]
- Zhu, Z.D.; Bai, B.F.; You, O.B.; Li, Q.Q.; Fan, S.S. Fano resonance boosted cascaded optical field enhancement in a plasmonic nanoparticle-in-cavity nanoantenna array and its SERS application. Light Sci. Appl. 2015, 4, e296. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.P.; Tong, L.M. Functionalized polymer nanofibers: A versatile platform for manipulating light at the nanoscale. Light Sci. Appl. 2013, 2, e102. [Google Scholar] [CrossRef]
- Hunt, S.T.; Milina, M.; Alba-Rubio, A.C.; Hendon, C.H.; Dumesic, J.A.; Román-Leshkov, Y. Self-assembly of noble metal monolayers on transition metal carbide nanoparticle catalysts. Science 2016, 352, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Karabchevsky, A.; Mosayyebi, A.; Kavokin, A.V. Tuning the chemiluminescence of a luminol flow using plasmonic nanoparticles. Light Sci. Appl. 2016, 5, e16164. [Google Scholar] [CrossRef]
- Linnenbank, H.; Grynko, Y.; Forstner, J.; Linden, S. Second harmonic generation spectroscopy on hybrid plasmonic/dielectric nanoantennas. Light Sci. Appl. 2016, 5, e16013. [Google Scholar] [CrossRef]
- Blum, O.; Shaked, N.T. Prediction of photothermal phase signatures from arbitrary plasmonic nanoparticles and experimental verification. Light Sci. Appl. 2015, 4, e322. [Google Scholar] [CrossRef]
- Yang, J.; Lee, J.Y.; Too, H.P. Core-Shell Ag-Au Nanoparticles from Replacement Reaction in Organic Medium. J. Phys. Chem. B 2015, 109, 19208–19212. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Jiang, Y.H.; Liu, Y.; Zhang, X.L.; Wang, Y.X.; Zhang, Y.J.; Wang, J.; Li, W.; Cheng, X. Effects of SiO2 content on the structure and magnetic properties of L10-FePt nanoparticles synthesized by the sol-gel method. Mater. Lett. 2013, 91, 348–351. [Google Scholar] [CrossRef]
- Xing, G.Z.; Wang, Y.; Wong, J.I.; Shi, Y.M.; Huang, Z.X.; Li, S.; Yang, H.Y. Hybrid CuO/SnO2 nanocomposites: Towards cost-effective and high performance binder free lithium ion batteries anode materials. Appl. Phys. Lett. 2014, 105, 143905–143912. [Google Scholar] [CrossRef]
- Sun, J.H.; Sui, H.D.; Wang, Z.-W.; Wang, K.; Yan, Y.; Lu, Q.; Li, L. Preparation of europium-doped nano-TiO2 transparent photocatalyst emulsion and photocatalytic performance. Chin. Opt. 2017, 10, 760–767. [Google Scholar] [CrossRef]
- Xing, G.Z.; Wang, D.D.; Cheng, C.-J.; He, M.; Li, S.; Wu, T. Emergent ferromagnetism in ZnO/Al2O3 core-shell nanowires: Towards oxide spinterfaces. Appl. Phys. Lett. 2013, 103, 022402–022407. [Google Scholar] [CrossRef]
- Li, X.; Ji, M.; Wang, H.; Tu, G.; Wan, X.; Liu, J.; Liu, J.; Xu, M.; Zhang, J. Research progress of near-infrared photothermal conversion nanocrystals. Chin. Opt. 2017, 10, 541–554. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Q. Research of zinc oxide quantum dot light-emitting diodes based on preparation of chemical solutions. J. Liq. Cryst. Disp. 2016, 31, 635–642. [Google Scholar] [CrossRef]
- Chen, X.Y.; Tian, Z. Recent progress in terahertz dynamic modulation based on graphene. Chin. Opt. 2017, 10, 86–97. [Google Scholar] [CrossRef]
- Tong, L.; Zhang, M.L.; Wang, F.; Zhang, D.M.; Wang, G.P. Fabrication of optical waveguide amplifiers based on bonding-type NaYF4: Er nanoparticles-polymer. Chin. Opt. 2017, 10, 219–225. [Google Scholar] [CrossRef]
- Psilodimitrakopoulos, S.; Mouchliadis, L.; Paradisanos, I.; Lemonis, A.; Kioseoglou, G.; Stratakis, E. Ultrahigh-resolution nonlinear optical imaging of the armchair orientation in 2D transition metal dichalcogenides. Light Sci. Appl. 2018, 7, e18005. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Jiang, G.M.; Zhu, H.Y.; Guo, S.J.; Su, D.; Lu, G.; Sun, S.H. Tuning nanoparticle structure and surface strain for catalysis optimization. J. Am. Chem. Soc. 2014, 136, 7734–7739. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tao, C.; Xiao, G.; Wei, G.; Li, L.; Liu, C.; Su, H. Controlled synthesis and photocatalysis of sea urchin-like Fe3O4@TiO2@Ag nanocomposites. Nanoscale 2016, 8, 5313–5326. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.Y.; Puig, T.; Obradors, X.; Ricart, S.; Ros, J. Ultra-fast microwave-assisted reverse microemulsion synthesis of Fe3O4@SiO2 core-shell nanoparticles as a highly recyclable silver nanoparticle catalytic platform in the reduction of 4-nitroaniline. RSC Adv. 2016, 6, 88762–88769. [Google Scholar] [CrossRef]
- Huo, J.; Zeng, H. Silver nanoparticles-sensitized cobalt complex for highly-efficient photocatalytic activity. Appl. Catal. B Environ. 2016, 199, 342–349. [Google Scholar] [CrossRef]
- Lu, L.Y.; Wang, D.; Xu, X.G.; Wang, H.C.; Miao, J.; Jiang, Y. Low temperature magnetic hardening in self-assembled FePt/Ag core-shell nanoparticles. Mater. Chem. Phys. 2011, 129, 995–999. [Google Scholar] [CrossRef]
- Hu, R.; Zheng, M.X.; Wu, J.C.; Li, C.; Shen, D.Q.; Yang, D.; Li, L.; Ge, M.F.; Chang, Z.M.; Dong, W.F. Core-Shell Magnetic Gold Nanoparticles for Magnetic Field-Enhanced Radio-Photothermal Therapy in Cervical Cancer. Nanomaterials 2017, 7, 111. [Google Scholar] [CrossRef] [PubMed]
- Andreou, D.; Iordanidou, D.; Tamiolakis, I.; Armatas, G.S.; Lykakis, I.N. Reduction of Nitroarenes into Aryl Amines and N-Aryl hydroxylamines via Activation of NaBH4 and Ammonia-Borane Complexes by Ag/TiO2 Catalyst. Nanomaterials 2016, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guo, W.; Yan, Y.; Lee, S.; Wang, T. Label-free super-resolution imaging of adenoviruses by submerged microsphere optical nanoscopy. Light Sci. Appl. 2013, 2, e104. [Google Scholar] [CrossRef]
- Wang, D.D.; Wang, W.L.; Huang, M.Y.; Lek, A.; Lam, J.; Mai, Z.H. Failure Analysis Depa Failure mechanism analysis and process improvement on time-dependent dielectric breakdown of Cu/ultra-low-k dielectric based on complementary Raman and FTIR spectroscopy study. AIP Adv. 2014, 4, 077124–077134. [Google Scholar] [CrossRef]
- Yu, D.H.; Yu, X.; Wang, C.; Liu, X.C.; Xing, Y. Synthesis of Natural Cellulose-Templated TiO2/Ag Nanosponge Composites and Photocatalytic Properties. ACS Appl. Mater. Interfaces 2012, 4, 2781–2787. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.M.; Zhang, D.D.; Ming, L.F.; Jiao, Y.C.; Chen, F. Synergistic effect of interfacial lattice Ag+ and Ag0 clusters in enhancing the photocatalytic performance of TiO2. Phys. Chem. Chem. Phys. 2014, 16, 19358–19367. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Xing, G.Z.; Yan, F.; Yan, Y.S.; Li, S. Ferromagnetic (Mn, N)-codoped ZnO nanopillars array: Experimental and computational insights. Appl. Phys. Lett. 2014, 104, 022412. [Google Scholar] [CrossRef]
- Pincella, F.; Isozaki, K.; Miki, K. A visible light-driven plasmonic photocatalyst. Light Sci. Appl. 2014, 3, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Xing, G.Z.; Fang, X.S.; Zhang, Z.; Wang, D.D.; Huang, X.; Guo, J.; Liao, L.; Zheng, Z.; Xu, H.R.; Yu, T. Ultrathin single-crystal ZnO nanobelts: Ag-catalyzed growth and field emission property. Nanotechnology 2010, 21, 255701–255710. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qu, S. Absorption spectra and near-electric field enhancement effects of Au- and Ag-Fe3O4 dimers. Appl. Surf. Sci. 2014, 292, 1002–1008. [Google Scholar] [CrossRef]
- Zhang, X.; Song, L.; Cai, L.; Tian, X.; Zhang, Q.; Qi, X.; Zhou, W.; Zhang, N.; Yang, F.; Fan, Q.X.; et al. Optical visualization and polarized light absorption of the single-wall carbon nanotube to verify intrinsic thermal applications. Light Sci. Appl. 2015, 4, e318. [Google Scholar] [CrossRef]
- Xing, G.Z.; Yi, J.B.; Wang, D.D.; Liao, L.; Yu, T.; Shen, Z.X.; Huan, C.H.A.; Sum, T.C.; Ding, J.; Wu, T. Strong correlation between ferromagnetism and oxygen deficiency in Cr-doped In2O3-δ nanostructures. Phys. Rev. B 2009, 79, 174406–174415. [Google Scholar] [CrossRef]
- Xing, G.Z.; Yi, J.B.; WU, T. Comparative Study of Room-Temperature Ferromagnetism in Cu-Doped ZnO Nanowires Enhanced by Structural Inhomogeneity. Adv. Mater. 2008, 20, 3521–3527. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, C.; Wang, Y.; Zhou, S.; Liu, J. Gel-limited synthesis of dumbbell-like Fe3O4-Ag composite microspheres and their SERS applications. Nanoscale 2014, 6, 12618–12625. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Wang, Z.L.; Li, S.; Wang, R.M.; Song, Y.J. Surface and interface engineering of FePt/C nanocatalysts for electro-catalytic methanol oxidation: Enhanced activity and durability. Nanoscale 2017, 9, 4066–4075. [Google Scholar] [CrossRef] [PubMed]
- Krylova, G.; Giovanetti, L.J.; Requejo, F.G.; Dimitrijevic, N.M.; Prakapenka, A.; Shevchenko, E.V. Study of Nucleation and Growth Mechanism of the Metallic Nanodumbbells. J. Am. Chem. Soc. 2012, 134, 4384–4392. [Google Scholar] [CrossRef] [PubMed]
- Niedermaier, I.; Kolbeck, C.; Steinrück, H.P.; Maier, F. Dual analyzer system for surface analysis dedicated for angle-resolved photoelectron spectroscopy at liquid surfaces and interfaces. Rev. Sci. Instrum. 2016, 87, 045105. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.K.; Bahadur, D. Influence of excess Fe accumulation over the surface of FePt nanoparticles: Structural and magnetic properties. J. Appl. Phys. 2013, 113, 134303–134313. [Google Scholar] [CrossRef]
- Goon, I.Y.; Lai, L.M.H.; Lim, M.; Munroe, P.; Gooding, J.J.; Amal, R. Fabrication and Dispersion of Gold-Shell-Protected Magnetite Nanoparticles: Systematic Control Using Polyethyleneimine. Chem. Mater. 2009, 21, 673–681. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Xie, A.; Li, S.; Wang, X.; Cai, Y. A Simple Method To Construct Bifunctional Fe3O4/Au Hybrid Nanostructures and Tune Their Optical Properties in the Near-Infrared Region. J. Phys. Chem. C 2010, 114, 4297–4301. [Google Scholar] [CrossRef]
- Lopes, G.; Vargas, J.M.; Sharma, S.K.; Beron, F.; Pirota, K.R.; Knobel, M.; Rettori, C.; Zysler, R.D. Ag-Fe3O4 Dimer Colloidal Nanoparticles: Synthesis and Enhancement of Magnetic Properties. J. Phys. Chem. C 2010, 114, 10148–10152. [Google Scholar] [CrossRef]
- Freitas, M.; Sá, C.M.; Barroso, M.F.; Pereira, C.; De-Los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J.; Delerue-Matos, C. Highly Monodisperse Fe3O4@Au Superparamagnetic Nanoparticles as Reproducible Platform for Genosensing Genetically Modified Organisms. ACS Sens. 2016, 1, 1044–1053. [Google Scholar] [CrossRef]
- Lu, S.X.; Yu, J.Y.; Cheng, Y.Y.; Wang, Q.; Barras, A.; Xu, W.G.; Szunerits, S.; Cornu, D.; Boukherroub, R. Preparation of silver nanoparticles/polydopamine functionalized polyacrylonitrile fiber paper and its catalytic activity for the reduction 4-nitrophenol. Appl. Surf. Sci. 2017, 411, 163–169. [Google Scholar] [CrossRef]
- Zhang, K.H.; Wang, C.W.; Rong, Z.; Xiao, R.; Zhou, Z.; Wang, S.Q. Silver coated magnetic microflowers as efficient and recyclable catalysts for catalytic reduction. New J. Chem. 2017, 41, 14199–14208. [Google Scholar] [CrossRef]
- Khan, M.M.; Lee, J.; Cho, M.H. Au@TiO2 nanocomposites for the catalytic degradation of methyl orange and methylene blue: An electron relay effect. J. Ind. Eng. Chem. 2014, 20, 1584–1590. [Google Scholar] [CrossRef]
- Wysocka, I.; Kowalska, E.; Trzcinski, K.; Łapinski, M.; Nowaczyk, G.; Zielinska-Jurek, A. UV-Vis-Induced Degradation of Phenol over Magnetic Photocatalysts Modified with Pt, Pd, Cu and Au Nanoparticles. Nanomaterials 2018, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, X.G.; Qi, X.H.; Luo, F.; He, G.H. Shape-Controlled Synthesis of Magnetic Iron Oxide@SiO2-Au@C Particles with Core-Shell Nanostructures. Langmuir 2015, 31, 5190–5197. [Google Scholar] [CrossRef] [PubMed]










© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, Y.; Kou, Q.; Chen, Y.; Sun, Y.; Han, D.; Wang, D.; Lu, Z.; Chen, L.; Yang, J.; et al. Highly Efficient, Low-Cost, and Magnetically Recoverable FePt–Ag Nanocatalysts: Towards Green Reduction of Organic Dyes. Nanomaterials 2018, 8, 329. https://doi.org/10.3390/nano8050329
Liu Y, Zhang Y, Kou Q, Chen Y, Sun Y, Han D, Wang D, Lu Z, Chen L, Yang J, et al. Highly Efficient, Low-Cost, and Magnetically Recoverable FePt–Ag Nanocatalysts: Towards Green Reduction of Organic Dyes. Nanomaterials. 2018; 8(5):329. https://doi.org/10.3390/nano8050329
Chicago/Turabian StyleLiu, Yang, Yuanyuan Zhang, Qiangwei Kou, Yue Chen, Yantao Sun, Donglai Han, Dandan Wang, Ziyang Lu, Lei Chen, Jinghai Yang, and et al. 2018. "Highly Efficient, Low-Cost, and Magnetically Recoverable FePt–Ag Nanocatalysts: Towards Green Reduction of Organic Dyes" Nanomaterials 8, no. 5: 329. https://doi.org/10.3390/nano8050329
APA StyleLiu, Y., Zhang, Y., Kou, Q., Chen, Y., Sun, Y., Han, D., Wang, D., Lu, Z., Chen, L., Yang, J., & Xing, S. G. (2018). Highly Efficient, Low-Cost, and Magnetically Recoverable FePt–Ag Nanocatalysts: Towards Green Reduction of Organic Dyes. Nanomaterials, 8(5), 329. https://doi.org/10.3390/nano8050329