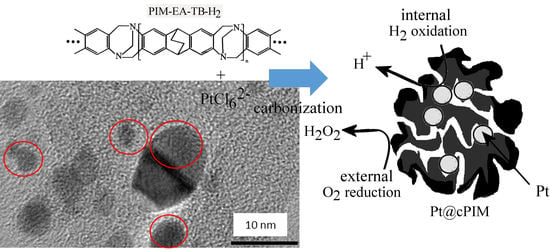
Platinum Nanoparticle Inclusion into a Carbonized Polymer of Intrinsic Microporosity: Electrochemical Characteristics of a Catalyst for Electroless Hydrogen Peroxide Production
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Instrumentation
2.3. Catalyst and Electrode Preparation
2.4. Catalyst Testing
3. Results and Discussion
3.1. Properties of Pt@cPIM I.: Structure and Porosity
3.2. Properties of Pt@cPIM II.: Electrochemical Characterization
3.3. Properties of Pt@cPIM III.: Catalysis and Hydrogen Peroxide Formation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lavacchi, A.; Miller, H.; Vizza, F. Nanotechnology in Electrocatalysis for Energy; Springer: New York, NY, USA, 2013; pp. 1–331. [Google Scholar]
- Guo, S.J.; Zhang, S.; Sun, S.H. Tuning nanoparticle catalysis for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2013, 52, 8526–8544. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Dinh, C.T.; Do, T.O. Tailoring the assembly, interfaces, and porosity of nanostructures toward enhanced catalytic activity. Chem. Commun. 2015, 51, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Madrid, E.; Lowe, J.P.; Msayib, K.J.; McKeown, N.B.; Song, Q.; Attard, G.A.; Düren, T.; Marken, F. Triphasic nature of polymers of intrinsic microporosity induces storage and catalysis effects in hydrogen and oxygen reactivity atelectrode surfaces. ChemElectroChem 2018. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, M.M.; Huang, H.; Liu, Y.; Kang, Z.H. Advances, challenges and promises of carbon dots. Inorg. Chem. Front. 2017, 4, 1963–1986. [Google Scholar] [CrossRef]
- Dumitrescu, I.; Unwin, P.R.; Macpherson, J.V. Electrochemistry at carbon nanotubes: Perspective and issues. Chem. Commun. 2009, 6886–6901. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.; Xia, F.J.; Arrowsmith, R.L.; Ge, H.B.; Nelson, G.W.; Foord, J.S.; Felipe-Sotelo, M.; Evans, N.D.M.; Mitchels, J.M.; Flower, S.E.; et al. Hydrothermal conversion of one-photon-fluorescent poly(4-vinylpyridine) into two-photon-fluorescent carbon nanodots. Langmuir 2014, 30, 11746–11752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, Y.Y.; He, D.P.; Sanchez-Fernandez, A.; Evans, C.; Edler, K.J.; Malpass-Evans, R.; Carta, M.; McKeown, N.B.; Clarke, T.J.; Taylor, S.H.; et al. Intrinsically microporous polymer retains porosity in vacuum thermolysis to electroactive heterocarbon. Langmuir 2015, 31, 12300–12306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, N.; Iniesta, J.; Leguey, V.M.; Armstrong, R.; Taylor, S.H.; Madrid, E.; Rong, Y.Y.; Castaing, R.; Malpass-Evans, R.; Carta, M. Carbonization of polymers of intrinsic microporosity to microporous heterocarbon: Capacitive pH measurements. Appl. Mater. Today 2017, 9, 136–144. [Google Scholar] [CrossRef] [Green Version]
- Qiu, S.L.; Ben, T. Polymers of instrinsic microporosity. In Porous Polymers: Design, Synthesis and Applications; Royal Society of Chemistry: Cambridge, UK, 2016. [Google Scholar]
- McKeown, N.B.; Budd, P.M. Polymers of intrinsic microporosity (PIMs): Organic materials for membrane separations, heterogeneous catalysis and hydrogen storage. Chem. Soc. Rev. 2006, 35, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Atilhan, M.; Anaya, B.; Al-Muhtaseb, S.; Aparicio, S.; Patel, H.; Thirion, D.; Yavuz, C.T. Investigation of ester-and amide-linker-based porous organic polymers for carbon dioxide capture and separation at wide temperatures and pressures. ACS Appl. Mater. Interfaces 2016, 8, 20772–20785. [Google Scholar] [CrossRef] [PubMed]
- Ramimoghadam, D.; Gray, E.M.; Webb, C.J. Review of polymers of intrinsic microporosity for hydrogen storage applications. Int. J. Hydrogen Energy 2016, 41, 16944–16965. [Google Scholar] [CrossRef]
- Polak-Krasna, K.; Dawson, R.; Holyfield, L.T.; Bowen, C.R.; Burrows, A.D.; Mays, T.J. Mechanical characterization of polymer of intrinsic microporosity PIM-1 for hydrogen storage applications. J. Mater. Sci. 2017, 52, 3862–3875. [Google Scholar] [CrossRef]
- Ghanem, B.S.; McKeown, N.B.; Budd, P.M.; Al-Harbi, N.M.; Fritsch, D.; Heinrich, K.; Starannikova, L.; Tokarev, A.; Yampolskii, Y. Synthesis, characterization, and gas permeation properties of a novel group of polymers with intrinsic microporosity: PIM-polyimides. Macromolecules 2009, 42, 7881–7888. [Google Scholar] [CrossRef]
- Rong, Y.Y.; Malpass-Evans, R.; Carta, M.; McKeown, N.B.; Attard, G.A.; Marken, F. High density heterogenization of molecular electrocatalysts in a rigid intrinsically microporous polymer. Electrochem. Commun. 2014, 46, 26–29. [Google Scholar] [CrossRef] [Green Version]
- Song, Q.L.; Cao, S.; Pritchard, R.H.; Ghalei, B.; Al-Muhtaseb, S.A.; Terentjev, E.M.; Cheetham, A.K.; Sivaniah, E. Controlled thermal oxidative crosslinking of polymers of intrinsic microporosity towards tunable molecular sieve membranes. Nat. Commun. 2014, 5, 12–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKeown, N.B.; Budd, P.M. Exploitation of intrinsic microporosity in polymer-based materials. Macromolecules 2010, 43, 5163–5176. [Google Scholar] [CrossRef]
- Thomas, A.; Kuhn, P.; Weber, J.; Titirici, M.M.; Antonietti, M. Porous polymers: Enabling solutions for energy applications. Macromol. Rapid Commun. 2009, 30, 221–236. [Google Scholar] [CrossRef] [PubMed]
- He, D.P.; Rong, Y.Y.; Kou, Z.K.; Mu, S.C.; Peng, T.; Malpass-Evans, R.; Carta, M.; McKeown, N.B.; Marken, F. Intrinsically microporous polymer slows down fuel cell catalyst corrosion. Electrochem. Commun. 2015, 59, 72–76. [Google Scholar] [CrossRef] [Green Version]
- Rong, Y.Y.; Malpass-Evans, R.; Carta, M.; McKeown, N.B.; Attard, G.A.; Marken, F. Intrinsically porous polymer protects catalytic gold particles for enzymeless glucose oxidation. Electroanalysis 2014, 26, 904–909. [Google Scholar] [CrossRef] [Green Version]
- Madrid, E.; Rong, Y.Y.; Carta, M.; McKeown, N.B.; Malpass-Evans, R.; Attard, G.A.; Clarke, T.J.; Taylor, S.H.; Long, Y.T.; Marken, F. Metastable ionic diodes derived from an amine-based polymer of intrinsic microporosity. Angew. Chem. Int. Ed. 2014, 53, 10751–10754. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.X.; Carta, M.; Malpass-Evans, R.; McKeown, N.B.; Madrid, E.; Marken, F. One-step preparation of microporous Pd@cPIM composite catalyst film for triphasic electrocatalysis. Electrochem. Commun. 2018, 86, 17–20. [Google Scholar] [CrossRef]
- Rong, Y.Y.; Song, Q.L.; Mathwig, K.; Madrid, E.; He, D.P.; Niemann, R.G.; Cameron, P.J.; Dale, S.E.C.; Bending, S.; Carta, M.; et al. Rectifier pH-induced reversal of ionic diode polarity in 300 nm thin membranes based on a polymer of intrinsic microporosity. Electrochem. Commun. 2016, 69, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Al-Kutubi, H.; Rassaei, L.; Olthuis, W.; Nelson, G.W.; Foord, J.S.; Holdway, P.; Carta, M.; Malpass-Evans, R.; McKeown, N.B.; Tsang, S.C.; et al. Polymers of intrinsic microporosity as high temperature templates for the formation of nanofibrous oxides. RSC Adv. 2015, 5, 73323–73326. [Google Scholar] [CrossRef] [Green Version]
- Carta, M.; Malpass-Evans, R.; Croad, M.; Rogan, Y.; Jansen, J.C.; Bernardo, P.; Bazzarelli, F.; McKeown, N.B. An efficient polymer molecular sieve for membrane gas separations. Science 2013, 339, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Salinas, O.; Ma, X.H.; Litwiller, E.; Pinnau, I. High-performance carbon molecular sieve membranes for ethylene/ethane separation derived from an intrinsically microporous polyimide. J. Membr. Sci. 2016, 500, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Kim, D.G.; Lee, K.; Baek, Y.; Yoo, Y.; Kim, Y.S.; Kim, B.G.; Lee, J.C. A carbonaceous membrane based on a polymer of intrinsic microporosity (PIM-1) for water treatment. Sci. Rep. 2016, 6, 36078. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.Y.; He, D.P.; Malpass-Evans, R.; Carta, M.; McKeown, N.B.; Gromboni, M.F.; Mascaro, L.H.; Nelson, G.W.; Foord, J.S.; Holdway, P.; et al. High-utilization nanoplatinum catalyst (Pt@cPIM) obtained via vacuum carbonization in a molecularly rigid polymer of intrinsic microporosity. Electrocatalysis 2017, 8, 132–143. [Google Scholar] [CrossRef]
- Xia, F.J.; Pan, M.; Mu, S.C.; Malpass-Evans, R.; Carta, M.; McKeown, N.B.; Attard, G.A.; Brew, A.; Morgan, D.J.; Marken, F. Polymers of intrinsic microporosity in electrocatalysis: Novel pore rigidity effects and lamella palladium growth. Electrochim. Acta 2014, 128, 3–9. [Google Scholar] [CrossRef] [Green Version]
- You, P.Y.; Kamarudin, S.K. Recent progress of carbonaceous materials in fuel cell applications: An overview. Chem. Eng. J. 2017, 309, 489–502. [Google Scholar] [CrossRef]
- Afraz, A.; Rafati, A.A.; Hajian, A.; Khoshnood, M. Electrodeposition of Pt nanoparticles on new porous graphitic carbon nanostructures prepared from biomass for fuel cell and methanol sensing applications. Electrocatalysis 2015, 6, 220–228. [Google Scholar] [CrossRef]
- Kakaei, K. Electrochemical characteristics and performance of platinum nanoparticles supported by Vulcan/polyaniline for oxygen reduction in PEMFC. Fuel Cells 2012, 12, 939–945. [Google Scholar] [CrossRef]
- Bernardo, P.; Scorzafave, V.; Clarizia, G.; Tocci, E.; Jansen, J.C.; Borgogno, A.; Malpass-Evans, R.; McKeown, N.B.; Carta, M.; Tasselli, F. Thin film composite membranes based on a polymer of intrinsic microporosity derived from Tröger’s base: A combined experimental and computational investigation of the role of residual casting solvent. J. Membr. Sci 2018, in press. [Google Scholar]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Berlin, Germany, 2004. [Google Scholar]
- Weber, J.; Wain, A.J.; Attard, G.A.; Marken, F. Electrothermal Annealing of Catalytic Platinum Microwire Electrodes: Towards Membrane-Free pH 7 Glucose Micro-Fuel Cells. Electroanalysis 2017, 29, 38–44. [Google Scholar] [CrossRef]
- Biegler, T.; Rand, D.A.J.; Woods, R. Limiting oxygen coverage on platinized platinum; relevance to determination of real platinum area by hydrogen adsorption. J. Electroanal. Chem. 1971, 29, 269–272. [Google Scholar] [CrossRef]
- Mtukula, A.C.; Bo, X.J.; Guo, L.P. Highly active non-precious metal electrocatalyst for the hydrogen evolution reaction based on nitrogen-doped graphene supported MoO2/WN/Mo2N. J. Alloys Compd. 2017, 692, 614–621. [Google Scholar] [CrossRef]
- Zhou, W.J.; Jia, J.; Lu, J.; Yang, L.J.; Hou, D.M.; Li, G.Q.; Chen, S.W. Recent developments of carbon-based electrocatalysts for hydrogen evolution reaction. Nano Energy 2016, 28, 29–43. [Google Scholar] [CrossRef]
- Yu, Z.T.; Ye, J.B.; Chen, W.X.; Xu, S.R. Fabrication of MoS2/reduced graphene oxide hybrid as an earth-abundant hydrogen evolution electrocatalyst. Mater. Lett. 2017, 188, 48–51. [Google Scholar] [CrossRef]
- Sheng, W.C.; Gasteiger, H.A.; Shao-Horn, Y. Hydrogen oxidation and evolution reaction kinetics on platinum: Acid vs. alkaline electrolytes. J. Electrochem. Soc. 2010, 157, B1529–B1536. [Google Scholar] [CrossRef]
- Wang, D.Z.; Shen, Y.L.; Zhang, X.Y.; Wu, Z.Z. Enhanced hydrogen evolution from the MoP/C hybrid by the modification of Ketjen black. J. Mater. Sci. 2017, 52, 3337–3343. [Google Scholar] [CrossRef]
- Hotchen, C.E.; Attard, G.A.; Bull, S.D.; Marken, F. One-step electroless growth of nano-fibrous platinum catalyst from “paint-on” PtCl62-solution in poly-(ethylene-glycol). Electrochim. Acta 2014, 137, 484–488. [Google Scholar] [CrossRef] [Green Version]
- Candelaria, S.L.; Bedford, N.M.; Woehl, T.J.; Rentz, N.S.; Showalter, A.R.; Pylypenko, S.; Bunker, B.A.; Lee, S.; Reinhart, B.; Ren, Y.; Ertem, S.P.; et al. Multi-component Fe-Ni hydroxide nanocatalyst for oxygen evolution and methanol oxidation reactions under alkaline conditions. ACS Catal. 2017, 7, 365–379. [Google Scholar] [CrossRef]
- Yang, C.Z.; van der Laak, N.K.; Chan, K.Y.; Zhang, X. Microwave-assisted microemulsion synthesis of carbon supported Pt-WO3 nanoparticles as an electrocatalyst for methanol oxidation. Electrochim. Acta 2012, 75, 262–272. [Google Scholar] [CrossRef]
- He, D.P.; Rong, Y.Y.; Carta, M.; Malpass-Evans, R.; McKeown, N.B.; Marken, F. Fuel cell anode catalyst performance can be stabilized with a molecularly rigid film of polymers of intrinsic microporosity (PIM). RSC Adv. 2016, 6, 9315–9319. [Google Scholar] [CrossRef]
- Gupta, S.; Qiao, L.; Zhao, S.; Xu, H.; Lin, Y.; Devaguptapu, S.V.; Wang, X.L.; Swihart, M.T.; Wu, G. Highly active and stable graphene tubes decorated with FeCoNi alloy nanoparticles via a template-free graphitization for bifunctional oxygen reduction and evolution. Adv. Energy Mater. 2016, 6, 12–14. [Google Scholar] [CrossRef]
- Wu, H.J.; Guo, C.Z.; Li, J.Q.; Ma, Z.L.; Feng, Q.Y.; Chen, C.G. A graphene-based electrocatalyst co-doped with nitrogen and cobalt for oxygen reduction reaction. Int. J. Hydrogen Energy 2016, 41, 20494–20501. [Google Scholar] [CrossRef]
- Song, W.Q.; Ren, Z.; Chen, S.Y.; Meng, Y.T.; Biswas, S.; Nandi, P.; Elsen, H.A.; Gao, P.X.; Suib, S.L. Ni- and Mn-promoted mesoporous Co3O4: A stable bifunctional catalyst with surface-structure-dependent activity for oxygen reduction reaction and oxygen evolution reaction. ACS Appl. Mater. Interfaces 2016, 8, 20802–20813. [Google Scholar] [CrossRef] [PubMed]
- Osgood, H.; Devaguptapu, S.V.; Xu, H.; Cho, J.; Wu, G. Transition metal (Fe, Co, Ni, and Mn) oxides for oxygen reduction and evolution bifunctional catalysts in alkaline media. Nano Today 2016, 11, 601–625. [Google Scholar] [CrossRef]
- Antoine, O.; Durand, R. RRDE study of oxygen reduction on Pt nanoparticles inside Nafion: H2O2 production in PEMFC cathode conditions. J. Appl. Electrochem. 2000, 30, 839–844. [Google Scholar] [CrossRef]
- Akram, A.; Freakley, S.J.; Reece, C.; Piccinini, M.; Shaw, G.; Edwards, J.K.; Desmedt, F.; Miquel, P.; Seuna, E.; Willock, D.J.; et al. Gas phase stabilizer-free production of hydrogen peroxide using supported gold-palladium catalysts. Chem. Sci. 2016, 7, 5833–5837. [Google Scholar] [CrossRef]
- Edwards, J.K.; Pritchard, J.; Lu, L.; Piccinini, M.; Shaw, G.; Carley, A.F.; Morgan, D.J.; Kiely, C.J.; Hutchings, G.J. The direct synthesis of hydrogen peroxide using platinum-promoted gold–palladium catalysts. Angew. Chem. Int. Ed. 2014, 53, 2381–2384. [Google Scholar] [CrossRef] [PubMed]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamik, R.K.; Hernández-Ibáñez, N.; Iniesta, J.; Edwards, J.K.; Howe, A.G.R.; Armstrong, R.D.; Taylor, S.H.; Roldan, A.; Rong, Y.; Malpass-Evans, R.; et al. Platinum Nanoparticle Inclusion into a Carbonized Polymer of Intrinsic Microporosity: Electrochemical Characteristics of a Catalyst for Electroless Hydrogen Peroxide Production. Nanomaterials 2018, 8, 542. https://doi.org/10.3390/nano8070542
Adamik RK, Hernández-Ibáñez N, Iniesta J, Edwards JK, Howe AGR, Armstrong RD, Taylor SH, Roldan A, Rong Y, Malpass-Evans R, et al. Platinum Nanoparticle Inclusion into a Carbonized Polymer of Intrinsic Microporosity: Electrochemical Characteristics of a Catalyst for Electroless Hydrogen Peroxide Production. Nanomaterials. 2018; 8(7):542. https://doi.org/10.3390/nano8070542
Chicago/Turabian StyleAdamik, Robert K., Naiara Hernández-Ibáñez, Jesus Iniesta, Jennifer K. Edwards, Alexander G. R. Howe, Robert D. Armstrong, Stuart H. Taylor, Alberto Roldan, Yuanyang Rong, Richard Malpass-Evans, and et al. 2018. "Platinum Nanoparticle Inclusion into a Carbonized Polymer of Intrinsic Microporosity: Electrochemical Characteristics of a Catalyst for Electroless Hydrogen Peroxide Production" Nanomaterials 8, no. 7: 542. https://doi.org/10.3390/nano8070542
APA StyleAdamik, R. K., Hernández-Ibáñez, N., Iniesta, J., Edwards, J. K., Howe, A. G. R., Armstrong, R. D., Taylor, S. H., Roldan, A., Rong, Y., Malpass-Evans, R., Carta, M., McKeown, N. B., He, D., & Marken, F. (2018). Platinum Nanoparticle Inclusion into a Carbonized Polymer of Intrinsic Microporosity: Electrochemical Characteristics of a Catalyst for Electroless Hydrogen Peroxide Production. Nanomaterials, 8(7), 542. https://doi.org/10.3390/nano8070542