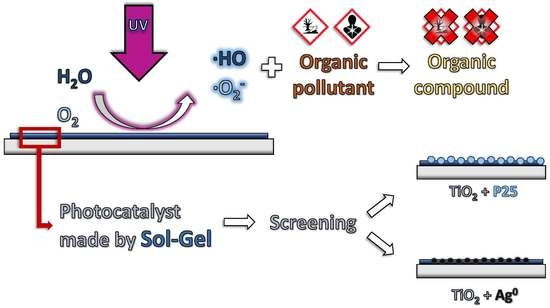
Sol-gel Syntheses of Photocatalysts for the Removal of Pharmaceutical Products in Water
Abstract
:Highlights
- Aqueous and organic synthesis pathways by sol-gel process for photocatalysts.
- Photodegradation of organic molecules in aqueous media.
- Ag NP-doped TiO2 (anatase) promising for the degradation of pharmaceutical compounds.
1. Introduction
2. Experimental
2.1. Material Preparation
2.1.1. Aqueous Sol-gel Synthesis
2.1.2. Organic Sol-gel Synthesis
2.1.3. Preparation of Thin Films
2.1.4. Powder Preparation
2.2. Film Characterization
2.3. Powder Characterization
2.4. Photocatalytic Degradation of Methylene Blue
2.5. Photocatalytic Degradation of Pharmaceutical Products
2.6. Toxicity Tests
3. Results and Discussion
3.1. Composition of Samples
3.2. Coating Aspect and Layer Thicknesses
3.3. Crystallographic Phases Composition
3.4. Diffuse Reflectance for Band-gap Measurement
3.5. Methylene Blue and Pharmaceutical Products Degradation
3.6. Toxicity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jobling, S.; Casey, D.; Rodgers-Gray, T.; Oehlmann, J.; Schulte-Oehlmann, U.; Pawlowski, S.; Baunbeck, T.; Turner, A.P.; Tyler, C.R. Comparative responses of molluscs and fish to environmental estrogens and an estrogenic effluent. Aquat. Toxicol. 2003, 65, 205–220. [Google Scholar] [CrossRef]
- Sumpter, J.P. Feminized responses in fish to environmental estrogens. Toxicol. Lett. 1995, 82–83, 737–742. [Google Scholar] [CrossRef]
- Sumpter, J.P.; Jobling, S. Vitellogenesis as a Biomarker for Estrogenic Contamination of the Aquatic Environment. Environ. Health Perspect. 2011, 103 (Suppl. 7), 173–178. [Google Scholar] [CrossRef]
- Bhandari, R.K.; Deem, S.L.; Holliday, D.K.; Jandegian, C.M.; Kassotis, C.D.; Nagel, S.C.; Tillitt, D.E.; vom Saal, F.S.; Rosenfeld, C.S. Effects of the environmental estrogenic contaminants bisphenol A and 17-alpha-ethinyl estradiol on sexual development and adult behaviors in aquatic wildlife species. Gen. Comp. Endocrinol. 2015, 214, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Amador, P.P.; Fernandes, R.M.; Prudêncio, M.C.; Barreto, M.P.; Duarte, I.M. Antibiotic resistance in wastewater: Occurrence and fate of Enterobacteriaceae producers of Class A and Class C β-lactamases. J. Environ. Sci. Health A Toxic Hazard. Subst. Environ. Eng. 2015, 50, 26–39. [Google Scholar] [CrossRef]
- Moges, F.; Endris, M.; Belyhun, Y.; Worku, W. Isolation and characterization of multiple drug resistance bacterial pathogens from waste water in hospital and non-hospital environments. Northwest Ethiop. BMC Res. Notes 2014, 7, 215. [Google Scholar] [CrossRef]
- Saif, A.-B.; Mahmoud, I.Y.; Paulson, J.R.; Al-Musharafi, S.K. Survival and Growth of Bacteria in Treated Wastewater and Water Distribution Systems and their Implication in Human Health: A review. Int. Arab. J. Antimicrob. Agents 2014, 4, 1–11. [Google Scholar] [CrossRef]
- Katouli, M.; Thompson, J.M.; Gündoğdu, A.; Stratton, H.M. Antibiotic resistant bacteria in hospital wastewaters and sewage treatment plants. In Science Forum and Stakeholder Engagement: Building Linkages, Collaboration and Science Quality; Griffith University: Brisbane, Australia, 2012; pp. 225–229. [Google Scholar]
- Rauf, M.A.; Ashraf, S.S. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J. 2009, 151, 10–18. [Google Scholar] [CrossRef]
- Sousa, M.A.; Gonçalves, C.; Vilar, V.J.P.; Boaventura, R.A.R.; Alpendurada, M.F. Suspended TiO2-assisted photocatalytic degradation of emerging contaminants in a municipal WWTP effluent using a solar pilot plant with CPCs. Chem. Eng. J. 2012, 198–199, 301–309. [Google Scholar] [CrossRef]
- Sousa, M.A.; Gonçalves, C.; Pereira, J.H.O.S.; Vilar, V.J.P.; Boaventura, R.A.R.; Alpendurada, M.F. Photolytic and TiO2-assisted photocatalytic oxidation of the anxiolytic drug lorazepam (Lorenin® pills) under artificial UV light and natural sunlight: A comparative and comprehensive study. Sol. Energy 2013, 87, 219–228. [Google Scholar] [CrossRef]
- Reinosa, J.J.; Docio CM, Á.; Ramírez, V.Z.; Lozano, J.F.F. Hierarchical nano ZnO-micro TiO2 composites: High UV protection yield lowering photodegradation in sunscreens. Ceram. Int. 2018, 44, 2827–2834. [Google Scholar] [CrossRef]
- Fujishima, A.; Hashimoto, K.; Watanabe, T. TiO2 Photocatalysis: Fundamentals and Applications; BKC, Inc.: Tokyo, Japan, 1999. [Google Scholar]
- Mahy, J.G.; Léonard, G.L.M.; Pirard, S.; Wicky, D.; Daniel, A.; Archambeau, C.; Liquet, D.; Heinrichs, B. Aqueous sol-gel synthesis and film deposition methods for the large-scale manufacture of coated steel with self-cleaning properties. J. Sol-Gel Sci. Technol. 2017, 81, 27–35. [Google Scholar] [CrossRef]
- Malengreaux, C.M.; Léonard, G.L.-M.; Pirard, S.L.; Cimieri, I.; Lambert, S.D.; Bartlett, J.R.; Heinrichs, B. How to modify the photocatalytic activity of TiO2 thin films through their roughness by using additives. A relation between kinetics, morphology and synthesis. Chem. Eng. J. 2014, 243, 537–548. [Google Scholar] [CrossRef]
- Braconnier, B.; Páez, C.A.; Lambert, S.; Alié, C.; Henrist, C.; Poelman, D.; Pirard, J.P.; Cloots, R.; Heinrichs, B. Ag- and SiO2-doped porous TiO2 with enhanced thermal stability. Microporous Mesoporous Mater. 2009, 122, 247–254. [Google Scholar] [CrossRef]
- Bodson, C.J.; Heinrichs, B.; Tasseroul, L.; Bied, C.; Mahy, J.G.; Wong Chi Man, M.; Lambert, S.D. Efficient P- and Ag-doped titania for the photocatalytic degradation of waste water organic pollutants. J. Alloys Compd. 2016, 682, 144–153. [Google Scholar] [CrossRef]
- Reinosa, J.J.; Leret, P.; Álvarez-Docio, C.M.; Del Campo, A.; Fernández, J.F. Enhancement of UV absorption behavior in ZnO-TiO2composites. Boletín de la Sociedad Española de Cerámica y Vidrio 2016, 55, 55–62. [Google Scholar] [CrossRef]
- Brinker, C.; Scherer, G. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing. Adv. Mater. 1990. [Google Scholar] [CrossRef]
- Bodson, C.J.; Lambert, S.D.; Alié, C.; Cattoën, X.; Pirard, J.P.; Bied, C.; Wong Chi Man, M.; Heinrichs, B. Effects of additives and solvents on the gel formation rate and on the texture of P- and Si-doped TiO2 materials. Microporous Mesoporous Mater. 2010, 134, 157–164. [Google Scholar] [CrossRef]
- European Commission. Directive 2008/105/EC of 16 December 2008 on environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/ECC, 86/280/ECC and amending Directive 2000/60/EC. Off. J. Eur. Union 2008, L348, 84–97. [Google Scholar]
- Ohno, T.; Sarukawa, K.; Tokieda, K.; Matsumura, M. Morphology of a TiO2 Photocatalyst (Degussa, P-25) Consisting of Anatase and Rutile Crystalline Phases. J. Catal. 2001, 203, 82–86. [Google Scholar] [CrossRef]
- Portehault, D.; Cassaignon, S.; Baudrin, E.; Jolivet, J.-P. Structural and morphological control of manganese oxide nanoparticles upon soft aqueous precipitation through MnO4−/Mn2+ reaction. J. Mater. Chem. 2009, 19, 2407. [Google Scholar] [CrossRef]
- Malengreaux, C.M.; Douven, S.; Poelman, D.; Heinrichs, B.; Bartlett, J.R. An ambient temperature aqueous sol-gel processing of efficient nanocrystalline doped TiO2-based photocatalysts for the degradation of organic pollutants. J. Sol-Gel Sci. Technol. 2014, 71, 557–570. [Google Scholar] [CrossRef]
- Bartlett, J.R.; Mol, S.D.C.; Woolfrey, J.L. The Structure of Multicomponent (Titania/Zirconia). Nanoparticles 1998, 118, 113–118. [Google Scholar] [CrossRef]
- Malengreaux, C. Modified TiO2-Based Photocatalytic Films and Powders Produced by Aqueous and Non-Aqueous Sol-Gel Processes for Water Purification. Ph.D. Thesis, University of Liège, Liège, Belgium, 2013. [Google Scholar]
- Legrand-Buscema, C.; Malibert, C.; Bach, S. Elaboration and characterization of thin films of TiO2 prepared by sol-gel process. Thin Solid Films 2002, 418, 79–84. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Yang, J.; Mei, S.; Ferreira, J.M.F.; Norby, P.; Quaresmâ, S. Fabrication of rutile rod-like particle by hydrothermal method: An insight into HNO3 peptization. J. Colloid Interface Sci. 2005, 283, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Léonard, G.L.-M.; Pàez, C.A.; Ramírez, A.E.; Mahy, J.G.; Heinrichs, B. Interactions between Zn2+ or ZnO with TiO2 to produce an efficient photocatalytic, superhydrophilic and aesthetic glass. J. Photochem. Photobiol. A Chem. 2018, 350, 32–43. [Google Scholar] [CrossRef]
- Páez, C.A.; Poelman, D.; Pirard, J.P.; Heinrichs, B. Unpredictable photocatalytic ability of H2-reduced rutile-TiO2 xerogel in the degradation of dye-pollutants under UV and visible light irradiation. Appl. Catal. B Environ. 2010, 94, 263–271. [Google Scholar] [CrossRef]
- Lambert, S.; Alié, C.; Pirard, J.P.; Heinrichs, B. Study of textural properties and nucleation phenomenon in Pd/SiO2, Ag/SiO2 and Cu/SiO2 cogelled xerogel catalysts. J. Non-Cryst. Solids 2004, 342, 70–81. [Google Scholar] [CrossRef]
- Choo, H.P.; Liew, K.Y.; Liu, H. Factors affecting the size of polymer stabilized Pd nanoparticles. J. Mater. Chem. 2002, 12, 934–937. [Google Scholar] [CrossRef]
- Flores, J.C.; Torres, V.; Popa, M.; Crespo, D.; Calderón-Moreno, J.M. Variations in morphologies of silver nanoshells on silica spheres, Colloids Surfaces A Physicochem. Eng. Asp. 2008, 330, 86–90. [Google Scholar] [CrossRef]
- Znaidi, L.; Chauveau, T.; Tallaire, A.; Liu, F.; Rahmani, M.; Bockelee, V.; Vrel, D.; Doppelt, P. Textured ZnO thin films by sol-gel process: Synthesis and characterizations. Thin Solid Films 2015, 617, 156–160. [Google Scholar] [CrossRef]
- Znaidi, L. Sol-gel-deposited ZnO thin films: A review. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2010, 174, 18–30. [Google Scholar] [CrossRef]
- Harris, D.C. Quantitative Chemical Analysis, 6th ed.; W.H. Freeman and Company: New York, NY, USA, 2003. [Google Scholar]
- Yu, J.; Zhao, X.; Zhao, Q. Photocatalytic activity of nanometer TiO2 thin films prepared by the sol-gel method. Mater. Chem. Phys. 2001, 69, 25–29. [Google Scholar] [CrossRef]
- Eufinger, K.; Poelman, D.; Poelman, H.; De Gryse, R.; Marin, G.B. Photocatalytic activity of dc magnetron sputter deposited amorphous TiO2 thin films. Appl. Surf. Sci. 2007, 254, 148–152. [Google Scholar] [CrossRef]
- Yu, J.-G.; Yu, H.-G.; Cheng, B.; Zhao, X.-J.; Yu, J.C.; Ho, W.-K. The Effect of Calcination Temperature on the Surface Microstructure and Photocatalytic Activity of TiO2 Thin Films Prepared by Liquid Phase Deposition. J. Phys. Chem. 2003, 107, 13871–13879. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol-gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61. [Google Scholar] [CrossRef]
- Tan, S.T.; Chen, B.J.; Sun, X.W.; Fan, W.J.; Kwok, H.S.; Zhang, X.H.; Chua, S.J. Blueshift of optical band gap in ZnO thin films grown by metal-organic chemical-vapor deposition. Appl. Phys. Lett. 2005, 98, 13505–41301. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chern, J.-M. Kinetics of Photocatalytic Decomposition of Methylene Blue. Ind. Eng. Chem. Res. 2006, 45, 6450–6457. [Google Scholar] [CrossRef]
- An, T.; Yang, H.; Song, W.; Li, G.; Luo, H.; Cooper, W.J. Mechanistic Considerations for the Advanced Oxidation Treatment of Fluoroquinolone Pharmaceutical Compounds using TiO2 Heterogeneous Catalysis. J. Phys. Chem. A 2010, 114, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Iliev, V.; Tomova, D.; Bilyarska, L.; Eliyas, A.; Petrov, L. Photocatalytic properties of TiO2 modified with platinum and silver nanoparticles in the degradation of oxalic acid in aqueous solution. Appl. Catal. B Environ. 2006, 63, 266–271. [Google Scholar] [CrossRef]

| Sample | Theoretical Dopant Content (wt%) | Actual Dopant Content (wt%) | Deposited Layers | Coating Thickness (nm) a | Absolute Roughness (nm) a | Scherrer Crystallite Size (nm) |
|---|---|---|---|---|---|---|
| TiO2 org (a) | - b | - b | 2 | 57 ± 15 | 0.8 ± 0.3 | 17 |
| TiO2 + Ag | 1 | 0.92 | 2 | 66 ± 3 | 2.0 ± 0.2 | 109 |
| TiO2 + P25 (a) | - b | - b | 3 | 97 ± 25 | 155 ± 33 | 18 |
| TiO2 + MnO2 | 5.42 | 3.49 | 2 | 89 ± 23 | 29.4 ± 8.4 | 18 |
| ZnO (b) | - b | - b | 2 | 56 ± 14 | 2.6 ± 0.4 | 80 |
| TiO2 aq (a) | - b | - b | 2 | 57 ± 10 | 1.5 ± 1.0 | - c |
| TiO2 + Zn2+ | 0.41 | 0.47 | 4 | 20 ± 4 | 0.7 ± 0.4 | - c |
| Sample | Direct Band Gap Energy (eV) | Indirect Band Gap Energy (eV) |
|---|---|---|
| TiO2 org | 3.14 | 2.95 |
| TiO2 + Ag | 3.14 | 2.82 |
| TiO2 + P25 | 3.13 | 2.93 |
| TiO2 + MnO2 | - a | - a |
| ZnO | - b | 3.04 |
| TiO2 aq | 3.24 | 2.87 |
| TiO2 + Zn2+ | 3.29 | 2.91 |
| Sample | dMB (%) | dlorazepam (%) | dtramadol (%) | dalprazolam (%) | dibuprofen (%) | dmetformin (%) | dPP a (%) |
|---|---|---|---|---|---|---|---|
| TiO2 org | 24.4 | 33.4 | 13.7 | 6.7 | 29.7 | 21.9 | 21.1 |
| TiO2 + Ag | 29.1 | 30.4 | 13.7 | 40.1 | 11.9 | 51.1 | 29.4 |
| TiO2 + P25 | 58.5 | 34.7 | 19.2 | 13.1 | 49.5 | 50.0 | 33.3 |
| TiO2 + MnO2 | 51.7 | 30.8 | 17.0 | 5.7 | 27.4 | 27.0 | 21.6 |
| ZnO | 36.5 | 7.0 | 14.2 | <1 | 19.8 | <1 | 8.2 |
| TiO2 aq | 37.1 | 31.4 | 4.0 | <1 | 14.1 | <1 | 9.9 |
| TiO2 + Zn2+ | 13.7 | 15.9 | <1 | <1 | <1 | 14.0 | 6.0 |
| Solution | Untreated | Treated with O3 | Treated with O3 + UV | Treated with O3 + UV + Photocatalysis |
|---|---|---|---|---|
| Toxicity | 1.32 | 1.33 | 1.33 | 1.19 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belet, A.; Wolfs, C.; Mahy, J.G.; Poelman, D.; Vreuls, C.; Gillard, N.; Lambert, S.D. Sol-gel Syntheses of Photocatalysts for the Removal of Pharmaceutical Products in Water. Nanomaterials 2019, 9, 126. https://doi.org/10.3390/nano9010126
Belet A, Wolfs C, Mahy JG, Poelman D, Vreuls C, Gillard N, Lambert SD. Sol-gel Syntheses of Photocatalysts for the Removal of Pharmaceutical Products in Water. Nanomaterials. 2019; 9(1):126. https://doi.org/10.3390/nano9010126
Chicago/Turabian StyleBelet, Artium, Cédric Wolfs, Julien G. Mahy, Dirk Poelman, Christelle Vreuls, Nathalie Gillard, and Stéphanie D. Lambert. 2019. "Sol-gel Syntheses of Photocatalysts for the Removal of Pharmaceutical Products in Water" Nanomaterials 9, no. 1: 126. https://doi.org/10.3390/nano9010126
APA StyleBelet, A., Wolfs, C., Mahy, J. G., Poelman, D., Vreuls, C., Gillard, N., & Lambert, S. D. (2019). Sol-gel Syntheses of Photocatalysts for the Removal of Pharmaceutical Products in Water. Nanomaterials, 9(1), 126. https://doi.org/10.3390/nano9010126