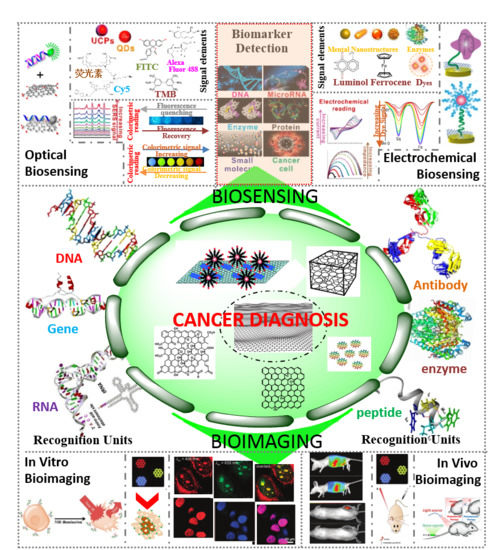
Biomarkers-based Biosensing and Bioimaging with Graphene for Cancer Diagnosis
Abstract
:1. Introduction
2. Graphene Nanomaterials
2.1. 2D Graphene Films
2.2. 3D Graphene Architectures
2.3. GHs Nanostructures
3. Surface Functionalization with Recognition Units
3.1. Antibody
3.2. Aptamer
4. Graphene Based Cancer Nanociagnosis
4.1. Biosensing
4.1.1. Electrochemical Biosensing
4.1.2. Optical Biosensing
4.2. Bioimaging
4.2.1. In Vitro Imaging
4.2.2. In-Vivo Imaging
5. Conclusion and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Tabish, T.A.; Zhang, S.; Winyard, P.G. Developing the next generation of graphene-based platforms for cancer therapeutics: The potential role of reactive oxygen species. Redox Biol. 2018, 15, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef] [PubMed]
- Rajeev, G.; Prieto Simon, B.; Marsal, L.F.; Voelcker, N.H. Advances in nanoporous anodic alumina-based biosensors to detect Biomarkers of clinical significance: A review. Adv. Healthc. Mater. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yi, X.; Wang, J.; Zhou, F. Electroanalytical and surface plasmon resonance sensors for detection of breast cancer and Alzheimer’s disease biomarkers in cells and body fluids. Analyst 2014, 139, 1814–1825. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2017, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Yang, Y.; Tian, J.; Shi, G. Photochemical synthesis of noble metal (Ag, Pd, Au, Pt) on graphene/ZnO multihybrid nanoarchitectures as electrocatalysis for H2O2 reduction. ACS Appl. Mater. Interfaces 2013, 5, 6762–6768. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Yu, Y.; Liu, X.; Ni, B.; Zhou, T.; Shi, G. Layer-by-layer self-assembly of functionalized graphene nanoplates for glucose sensing in vivo integrated with on-line microdialysis system. Biosens. Bioelectron. 2012, 32, 118–126. [Google Scholar] [CrossRef]
- Tang, H.; Cai, D.; Ren, T.; Xiong, P.; Liu, Y.; Gu, H.; Shi, G. Fabrication of a low background signal glucose biosensor with 3D network materials as the electrocatalyst. Anal. Biochem. 2019, 567, 63–71. [Google Scholar] [CrossRef]
- Shi, J.; Li, X.; Chen, Q.; Gao, K.; Song, H.; Guo, S.; Li, Q.; Fang, M.; Liu, W.; Liu, H.; et al. A monocrystal graphene domain biosensor array with differential output for real-time monitoring of glucose and normal saline. Nanoscale 2015, 7, 7867–7872. [Google Scholar] [CrossRef]
- Choi, B.G.; Park, H.S.; Park, T.J.; Yang, M.H.; Kim, J.S.; Jang, S.Y.; Heo, N.S.; Lee, S.Y.; Kong, J.; Hong, W.H. Solution chemistry of self-assembled graphene nanohybrids for high-performance flexible biosensors. ACS Nano 2010, 4, 2910–2918. [Google Scholar] [CrossRef]
- Cacciotti, I.; House, J.N.; Mazzuca, C.; Valentini, M.; Madau, F.; Palleschi, A.; Straffi, P.; Nanni, F. Neat and GNPs loaded natural rubber fibers by electrospinning: Manufacturing and characterization. Mater. Des. 2015, 88, 1109–1118. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, S.; Yang, C.F. Electrochemical biosensors for cancer biomarker detection. Electroanalysis 2012, 24, 2213–2229. [Google Scholar] [CrossRef]
- Viswambari, D.R.; Doble, M.; Verma, R.S. Nanomaterials for early detection of cancer biomarker with special emphasis on gold nanoparticles in immunoassays/sensors. Biosens. Bioelectron. 2015, 68, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.V.; Fernandes, B.F.; Bueno, P.R.; Davis, J.J. Graphene-based protein biomarker detection. Bioanalysis 2015, 7, 725–742. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Esimbekova, E.N.; Kratasyuk, V.A. Rapid biosensing tools for cancer biomarkers. Biosens. Bioelectron. 2017, 87, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Akiba, U.; Anzai, J.I. Recent progress in nanomaterial-based electrochemical biosensors for cancer biomarkers: A Review. Molecules 2017, 22, 1048. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.M.; Kang, J.H.; Hong, B.H. Graphene-based nanomaterials for versatile imaging studies. Chem. Soc. Rev. 2015, 44, 4835–4852. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, H.; Tang, J.; Deng, S.; Yan, F.; Li, W.; Qu, M. Carbon dots doped with heteroatoms for fluorescent bioimaging: A review. Microchim. Acta 2016, 184, 343–368. [Google Scholar] [CrossRef]
- Lin, J.; Chen, X.; Huang, P. Graphene-based nanomaterials for bioimaging. Adv. Drug Deliv. Rev. 2016, 105 Pt B, 242–254. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Feng, W.; Li, F. Water-soluble lanthanide upconversion nanophosphors: Synthesis and bioimaging applications in vivo. Coord. Chem. Rev. 2014, 273–274, 100–110. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Yang, Y.; Yuan, Q. Aptamer-functionalized carbon nanomaterials electrochemical sensors for detecting cancer relevant biomolecules. Carbon 2018, 129, 380–395. [Google Scholar] [CrossRef]
- Chen, L.; Hernandez, Y.; Feng, X.; Mullen, K. From nanographene and graphene nanoribbons to graphene sheets: Chemical synthesis. Angew. Chem. Int. Ed. 2012, 51, 7640–7654. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Nishina, N.; Enoki, T.; Aihara, J. Aromatic character of nanographene model compounds. J. Phys. Chem. A 2014, 118, 3014–3025. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Yang, H.H.; Zhu, C.L.; Chen, X.; Chen, G.N. A graphene platform for sensing biomolecules. Angew. Chem. Int. Ed. 2009, 48, 4785–4787. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene based electrochemical sensors and biosensors: A review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Qian, J.; Wang, D.; Cai, F.H.; Xi, W.; Peng, L.; Zhu, Z.F.; He, H.; Hu, M.L.; He, S. Observation of multiphoton-induced fluorescence from graphene oxide nanoparticles and applications in in vivo functional bioimaging. Angew. Chem. Int. Ed. 2012, 51, 10570–10575. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Qiao, C.; Tang, S.; Li, Y.; Yuan, W.; Li, B.; Tian, L.; Liu, F.; Hu, R.; et al. Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chem. Commun. 2011, 47, 6858–6860. [Google Scholar] [CrossRef]
- Zhu, Z.; Qian, J.; Zhao, X.; Qin, W.; Hu, R.; Zhang, H.; Li, D.; Xu, Z.; Tang, B.Z.; He, S. Stable and Size-Tunable Aggregation-Induced Emission Nanoparticles Encapsulated with Nanographene Oxide and Applications in Three-Photon Fluorescence Bioimaging. ACS Nano 2016, 10, 588–597. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Graphene quantum dots: Emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 2012, 48, 3686–3699. [Google Scholar] [CrossRef]
- Gao, H.; Duan, H. 2D and 3D graphene materials: Preparation and bioelectrochemical applications. Biosens. Bioelectron. 2015, 65, 404–419. [Google Scholar] [CrossRef]
- Xiao, X.; Beechem, T.E.; Brumbach, M.T.; Lambert, T.N.; Davis, D.J.; Michael, J.R.; Washburn, C.M.; Wang, J.; Brozik, S.M.; Wheeler, D.R.; et al. Lithographically defined three-dimensional graphene structures. J. Am. Chem. Soc. 2012, 6, 3573–3579. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Fan, Z. Design of advanced porous graphene materials: From graphene nanomesh to 3D architectures. Nanoscale 2014, 6, 1922–1945. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Lu, G.; Chen, J. Three-dimensional graphene-based composites for energy applications. Nanoscale 2015, 7, 6924–6943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.C.; Dong, X.C.; Chan-Park, M.B.; Song, H.; Chen, P. Macroporous and monolithic anode based on polyaniline hybridized three-dimensional graphene for high-performance microbial fuel cells. J. Am. Chem. Soc. 2012, 6, 2394–2400. [Google Scholar] [CrossRef]
- Liu, M.; Chen, P.Y.; Hurt, R.H. Graphene inks as versatile templates for printing tiled metal oxide crystalline films. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Sha, J.; Li, Y.; Villegas, S.R.; Wang, T.; Dong, P.; Ji, Y.; Lee, S.K.; Zhang, C.; Zhang, J.; Smith, R.H.; et al. Three-dimensional printed graphene foams. ACS Nano 2017, 11, 6860–6867. [Google Scholar] [CrossRef]
- Dong, X.C.; Xu, H.; Wang, X.W.; Huang Yi., X.; Chan-Park, M.B.; Zhang, H.; Wang, L.H.; Huang, W.; Chen, P. 3D graphene cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. J. Am. Chem. Soc. 2012, 6, 3206–3213. [Google Scholar] [CrossRef]
- Shi, Q.; Cha, Y.; Song, Y.; Lee, J.I.; Zhu, C.; Li, X.; Song, M.K.; Du, D.; Lin, Y. 3D graphene-based hybrid materials: Synthesis and applications in energy storage and conversion. Nanoscale 2016, 8, 15414–15447. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Todd, A.D.; Bielawski, C.W. Harnessing the chemistry of graphene oxide. Chem. Soc. Rev. 2014, 43, 5288–5301. [Google Scholar] [CrossRef]
- Deng, K.; Li, C.; Qiu, X.; Zhou, J.; Hou, Z. Synthesis of Cobalt hexacyanoferrate decorated graphene oxide/carbon nanotubes-COOH hybrid and their application for sensitive detection ofhydrazine. Electrochim. Acta 2015, 174, 1096–1103. [Google Scholar] [CrossRef]
- Lightcap, I.V.; Kosel, T.H.; Kamat, P.V. Anchoring semiconductor and metal nanoparticles on a two-dimensional catalyst mat. Storing and shuttling electrons with reduced graphene oxide. Nano Lett. 2010, 10, 577–583. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shan, C.; Li, F.; Han, D.; Zhang, Q.; Niu, L. Covalent functionalization of polydisperse chemically-converted graphene sheets with amine-terminated ionic liquid. Chem. Commun. 2009, 3880–3882. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yang, H.; Shan, C.; Zhang, Q.; Han, D.; Ivaska, A.; Niu, L. The synthesis of perylene-coated graphene sheets decorated with Au nanoparticles and its electrocatalysis toward oxygen reduction. J. Mater. Chem. 2009, 19, 4022. [Google Scholar] [CrossRef]
- Wang, D.; Choi, D.; Li, J.; Yang, Z.; Nie, Z.; Kou, R.; Hu, D.; Wang, C.; Laxmikant, V.S.; Zhang, J.; et al. Self-assembled TiO2–graphene hybrid nanostructures for enhanced Li-ion insertion. J. Am. Chem. Soc. 2009, 3, 907–914. [Google Scholar] [CrossRef]
- Topkaya, S.N.; Azimzadeh, M.; Ozsoz, M. Electrochemical biosensors for cancer biomarkers detection: Recent advances and challenges. Electroanalysis 2016, 28, 1402–1419. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent gunctionalization of hraphene and hraphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef]
- Alava, T.; Mann, J.A.; Theodore, C.; Benitez, J.J.; Dichtel, W.R.; Parpia, J.M.; Craighead, H.G. Control of the graphene-protein interface is required to preserve adsorbed protein function. Anal. Chem. 2013, 85, 2754–2759. [Google Scholar] [CrossRef]
- Mann, J.A.; Alava, T.; Craighead, H.G.; Dichtel, W.R. Preservation of antibody selectivity on graphene by conjugation to a tripod monolayer. Angew. Chem. Int. Ed. 2013, 52, 3177–3180. [Google Scholar] [CrossRef]
- Hong, H.; Yang, K.; Zhang, Y.; Engle, J.W.; Feng, L.; Yang, Y.; Nayak, T.R.; Goel, S.; Bean, J.; Theuer, C.P.; et al. In vivo targeting and imaging of tumor vasculature with radiolabeled, antibody-conjugated nanographene. J. Am. Chem. Soc. 2012, 6, 2361–2370. [Google Scholar] [CrossRef]
- Chang, Y.M.; Donovan, M.J.; Tan, W. Using aptamers for cancer biomarker discovery. J. Nucleic Acids 2013, 2013, 817350. [Google Scholar] [CrossRef]
- Azimzadeh, M.; Rahaie, M.; Nasirizadeh, N.; Ashtari, K.; Naderi-Manesh, H. An electrochemical nanobiosensor for plasma miRNA-155, based on graphene oxide and gold nanorod, for early detection of breast cancer. Biosens. Bioelectron. 2016, 77, 99–106. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Li, P.; Nie, Z.; Li, J. Applications of graphene and its derivatives in intracellular biosensing and bioimaging. Analyst 2016, 141, 4541–4553. [Google Scholar] [CrossRef]
- Geetha Bai, R.; Ninan, N.; Muthoosamy, K.; Manickam, S. Graphene: A versatile platform for nanotheranostics and tissue engineering. Prog. Mater. Sci. 2018, 91, 24–69. [Google Scholar] [CrossRef]
- Kirsch, J.; Siltanen, C.; Zhou, Q.; Revzin, A.; Simonian, A. Biosensor technology: Recent advances in threat agent detection and medicine. Chem. Soc. Rev. 2013, 42, 8733–8768. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Khanra, P.; Mishra, A.K.; Kim, N.H.; Lee, J.H. Recent advances in graphene-based biosensors. Biosens. Bioelectron. 2011, 26, 4637–4648. [Google Scholar] [CrossRef]
- Verma, S.; Singh, A.; Shukla, A.; Kaswan, J.; Arora, K.; Ramirez-Vick, J.; Singh, P.; Singh, S.P. Anti-IL8/AuNPs-rGO/ITO as an immunosensing platform for noninvasive electrochemical detection of oral cancer. ACS Appl. Mater. Interfaces 2017, 9, 27462–27474. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Su, H.; Mao, L.; Jiang, X.; Zhang, T.; Dai, X. Three-dimensional electrochemical DNA biosensor based on 3D graphene-Ag nanoparticles for sensitive detection of CYFRA21-1 in non-small cell lung cancer. Sens. Actuators B Chem. 2018, 255, 2910–2918. [Google Scholar] [CrossRef]
- Castillo, J.J.; Svendsen, W.E.; Rozlosnik, N.; Escobar, P.; Martínez, F.; Castillo-León, J. Detection of cancer cells using a peptidenanotube–folic acid modified graphene electrode. Analyst 2013, 138, 1026–1031. [Google Scholar] [CrossRef]
- Gupta, P.; Bharti, A.; Kaur, N.; Singh, S.; Prabhakar, N. An electrochemical aptasensor based on gold nanoparticles and graphene oxide doped poly(3,4-ethylenedioxythiophene) nanocomposite for detection of MUC1. J. Electroanal. Chem. 2018, 813, 102–108. [Google Scholar] [CrossRef]
- Jang, H.D.; Kim, S.K.; Chang, H.; Choi, J.W. 3D label-free prostate specific antigen (PSA) immunosensor based on graphene-gold composites. Biosens. Bioelectron. 2015, 63, 546–551. [Google Scholar] [CrossRef]
- Kong, F.Y.; Xu, M.T.; Xu, J.J.; Chen, H.Y. A novel lable-free electrochemical immunosensor for carcinoembryonic antigen based on gold nanoparticles-thionine-reduced graphene oxide nanocomposite film modified glassy carbon electrode. Talanta 2011, 85, 2620–2625. [Google Scholar] [CrossRef]
- Kumar, S.; Ashish; Kumar, S.; Augustine, S.; Yadav, S.; Yadav, B.K.; Chauhan, R.P.; Dewan, A.K.; Malhotra, B.D. Effect of Brownian motion on reduced agglomeration of nanostructured metal oxide towards development of efficient cancer biosensor. Biosens. Bioelectron. 2018, 102, 247–255. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Wang, Y.; Ma, H.; Du, B.; Wei, Q. Facile synthesis of cuprous oxide nanowires decorated graphene oxide nanosheets nanocomposites and its application in label-free electrochemical immunosensor. Biosens. Bioelectron. 2017, 87, 745–751. [Google Scholar] [CrossRef]
- Du, D.; Zou, Z.; Shin, Y.; Wang, J.; Wu, H.; Engelhard, M.H.; Liu, J.; Aksay, I.A.; Lin, Y. Sensitive immunosensor for cancer biomarker based on dual signal amplification strategy of graphene sheets and multienzyme functionalized carbon nanospheres. Anal. Chem. 2010, 82, 2989–2995. [Google Scholar] [CrossRef]
- Ren, X.; Ma, H.; Zhang, T.; Zhang, Y.; Yan, T.; Du, B.; Wei, Q. Sulfur-doped graphene-based immunological biosensing platform for multianalysis of cancer biomarkers. ACS Appl. Mater. Interfaces 2017, 9, 37637–37644. [Google Scholar] [CrossRef]
- Guo, Z.; Hao, T.; Du, S.; Chen, B.; Wang, Z.; Li, X.; Wang, S. Multiplex electrochemiluminescence immunoassay of two tumor markers using multicolor quantum dots as labels and graphene as conducting bridge. Biosens. Bioelectron. 2013, 44, 101–107. [Google Scholar] [CrossRef]
- Jia, X.; Chen, X.; Han, J.; Ma, J.; Ma, Z. Triple signal amplification using gold nanoparticles, bienzyme and platinum nanoparticles functionalized graphene as enhancers for simultaneous multiple electrochemical immunoassay. Biosens. Bioelectron. 2014, 53, 65–70. [Google Scholar] [CrossRef]
- Park, S.; Singh, A.; Kim, S.; Yang, H. Electroreduction-based electrochemical-enzymatic redox cycling for the detection of cancer antigen 15-3 using graphene oxide-modified indium-tin oxide electrodes. Anal. Chem. 2014, 86, 1560–1566. [Google Scholar] [CrossRef]
- Tang, J.; Tang, D.; Niessner, R.; Chen, G.; Knopp, D. Magneto-controlled graphene immunosensing platform for simultaneous multiplexed electrochemical immunoassay using distinguishable signal tags. Anal. Chem. 2011, 83, 5407–5414. [Google Scholar] [CrossRef]
- Wu, Y.; Xue, P.; Kang, Y.; Hui, K.M. Paper-based microfluidic electrochemical immunodevice integrated with nanobioprobes onto graphene film for ultrasensitive multiplexed detection of cancer biomarkers. Anal. Chem. 2013, 85, 8661–8668. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Wang, T.; Li, J. Positive potential operation of a cathodic electrogenerated chemiluminescence immunosensor based on luminol and graphene for cancer biomarker detection. Anal. Chem. 2011, 83, 3817–3823. [Google Scholar] [CrossRef]
- Yang, Z.; Luo, S.; Li, J.; Shen, J.; Yu, S.; Hu, X.; Dionysiou, D.D. A streptavidin functionalized graphene oxide/Au nanoparticles composite for the construction of sensitive chemiluminescent immunosensor. Anal. Chim. Acta 2014, 839, 67–73. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Z.; Cui, Y.; Chen, G.; Tang, D. Nanogold-enhanced graphene nanosheets as multienzyme assembly for sensitive detection of low-abundance proteins. Biosens. Bioelectron. 2013, 44, 108–114. [Google Scholar] [CrossRef]
- Chen, X.; Jia, X.; Han, J.; Ma, J.; Ma, Z. Electrochemical immunosensor for simultaneous detection of multiplex cancer biomarkers based on graphene nanocomposites. Biosens. Bioelectron. 2013, 50, 356–361. [Google Scholar] [CrossRef]
- Su, M.; Zhang, Y.; Song, X.; Ge, S.; Yan, M.; Yu, J.; Huang, J. Three-dimensional nanoflower-like MnO2 functionalized graphene as catalytically promoted nanolabels for ultrasensitive electrochemiluminescence immunoassay. Electrochim. Acta 2013, 97, 333–340. [Google Scholar] [CrossRef]
- Wang, D.; Gan, N.; Zhang, H.; Li, T.; Qiao, L.; Cao, Y.; Su, X.; Jiang, S. Simultaneous electrochemical immunoassay using graphene-Au grafted recombinant apoferritin-encoded metallic labels as signal tags and dual-template magnetic molecular imprinted polymer as capture probes. Biosens. Bioelectron. 2015, 65, 78–82. [Google Scholar] [CrossRef]
- Zhu, Q.; Chai, Y.; Yuan, R.; Zhuo, Y.; Han, J.; Li, Y.; Liao, N. Amperometric immunosensor for simultaneous detection of three analytes in one interface using dual functionalized graphene sheets integrated with redox-probes as tracer matrixes. Biosens. Bioelectron. 2013, 43, 440–445. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, N.; Ma, Z. Prussian blue-gold nanoparticles-ionic liquid functionalized reduced graphene oxide nanocomposite as label for ultrasensitive electrochemical immunoassay of alpha-fetoprotein. Anal. Chim. Acta 2014, 829, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Wang, Y.; Yang, H.; Yang, P.; Cheng, X.; Yan, M.; Yu, J. A photoelectrochemical biosensor using ruthenium complex-reduced graphene oxide hybrid as the photocurrent signal reporter assembled on rhombic TiO2 nanocrystals driven by visible light. Anal. Chim. Acta 2014, 828, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tian, J.; Zhao, Y.; Zhao, S. Ag/Au nanoparticles coated graphene electrochemical sensor for ultrasensitive analysis of carcinoembryonic antigen in clinical immunoassay. Sens. Actuators B Chem. 2015, 206, 570–576. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Yu, J.; Ge, S.; Song, X. All-graphene composite materials for signal amplification toward ultrasensitive electrochemical immunosensing of tumor marker. Biosens. Bioelectron. 2015, 71, 108–114. [Google Scholar] [CrossRef]
- Li, N.; Ma, H.; Cao, W.; Wu, D.; Yan, T.; Du, B.; Wei, Q. Highly sensitive electrochemical immunosensor for the detection of alpha fetoprotein based on PdNi nanoparticles and N-doped graphene nanoribbons. Biosens. Bioelectron. 2015, 74, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Heidari, R.; Rashidiani, J.; Abkar, M.; Taheri, R.A.; Moghaddam, M.M.; Mirhosseini, S.A.; Seidmoradi, R.; Nourani, M.R.; Mahboobi, M.; Keihan, A.H.; et al. CdS nanocrystals/graphene oxide-AuNPs based electrochemiluminescence immunosensor in sensitive quantification of a cancer biomarker. Biosens. Bioelectron. 2019, 126, 7–14. [Google Scholar] [CrossRef]
- Salahandish, R.; Ghaffarinejad, A.; Omidinia, E.; Zargartalebi, H.; Majidzadeh, A.K.; Naghib, S.M.; Sanati-Nezhad, A. Label-free ultrasensitive detection of breast cancer miRNA-21 biomarker employing electrochemical nano-genosensor based on sandwiched AgNPs in PANI and N-doped graphene. Biosens. Bioelectron. 2018, 120, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Tian, Q.; Xu, J.; Lu, L.; Ma, X.; Yu, Y. Aerogels prepared from polymeric beta-cyclodextrin and graphene aerogels as a novel host-guest system for immobilization of antibodies: A voltammetric immunosensor for the tumor marker CA 15-3. Miccrochim. Acta 2018, 185, 1–7. [Google Scholar]
- Akbarnia, A.; Zare, H.R. A voltammetric assay for microRNA-25 based on the use of amino-functionalized graphene quantum dots and ss- and ds-DNAs as gene probes. Microchim. Acta 2018, 185, 1–8. [Google Scholar] [CrossRef]
- Dong, W.; Ren, Y.; Bai, Z.; Yang, Y.; Wang, Z.; Zhang, C.; Chen, Q. Trimetallic AuPtPd nanocomposites platform on graphene: Applied to electrochemical detection and breast cancer diagnosis. Talanta 2018, 189, 79–85. [Google Scholar] [CrossRef]
- Shoja, Y.; Kermanpur, A.; Karimzadeh, F. Diagnosis of EGFR exon21 L858R point mutation as lung cancer biomarker by electrochemical DNA biosensor based on reduced graphene oxide/functionalized ordered mesoporous carbon/Ni-oxytetracycline metallopolymer nanoparticles modified pencil graphite electrode. Biosens. Bioelectron. 2018, 113, 108–115. [Google Scholar] [PubMed]
- Yang, S.; Zhang, F.; Wang, Z.; Liang, Q. A graphene oxide-based label-free electrochemical aptasensor for the detection of alpha-fetoprotein. Biosens. Bioelectron. 2018, 112, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, M.; Tagi, S.; Solhi, E.; Mokhtarzadeh, A.; Shadjou, N.; Eftekhari, A.; Mahboob, S. An innovative immunosensor for ultrasensitive detection of breast cancer specific carbohydrate (CA 15-3) in unprocessed human plasma and MCF-7 breast cancer cell lysates using gold nanospear electrochemically assembled onto thiolated graphene quantum dots. Int. J. Biol. Macromol. 2018, 114, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Chiu, N.F.; Lin, T.L.; Kuo, C.T. Highly sensitive carboxyl-graphene oxide-based surface plasmon resonance immunosensor for the detection of lung cancer for cytokeratin 19 biomarker in human plasma. Sens. Actuators B Chem. 2018, 265, 264–272. [Google Scholar] [CrossRef]
- Pachauri, N.; Dave, K.; Dinda, A.; Solanki, P.R. Cubic CeO2 implanted reduced graphene oxide-based highly sensitive biosensor for non-invasive oral cancer biomarker detection. J. Mater. Chem. B 2018, 6, 3000–3012. [Google Scholar] [CrossRef]
- Singh, V.K.; Kumar, S.; Pandey, S.K.; Srivastava, S.; Mishra, M.; Gupta, G.; Malhotra, B.D.; Tiwari, R.S.; Srivastava, A. Fabrication of sensitive bioelectrode based on atomically thin CVD grown graphene for cancer biomarker detection. Biosens. Bioelectron. 2018, 105, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Nie, G.; Wang, Y.; Tang, Y.; Zhao, D.; Guo, Q. A graphene quantum dots based electrochemiluminescence immunosensor for carcinoembryonic antigen detection using poly(5-formylindole)/reduced graphene oxide nanocomposite. Biosens. Bioelectron. 2018, 101, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Dighe, K.; Wang, Z.; Srivastava, I.; Daza, E.; Schwartz-Dual, A.S.; Ghannam, J.; Misra, S.K.; Pan, D. Detection of prostate specific antigen (PSA) in human saliva using an ultra-sensitive nanocomposite of graphene nanoplatelets with diblock-co-polymers and Au electrodes. Analyst 2018, 143, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, Z.; Fan, L.; Guo, Y. Electrochemical prostate specific antigen aptasensor based on hemin functionalized graphene-conjugated palladium nanocomposites. Microchim. Acta 2018, 185, 1–8. [Google Scholar] [CrossRef]
- Barman, S.C.; Hossain, M.F.; Yoon, H.; Park, J.Y. Trimetallic Pd@Au@Pt nanocomposites platform on -COOH terminated reduced graphene oxide for highly sensitive CEA and PSA biomarkers detection. Biosens. Bioelectron. 2018, 100, 16–22. [Google Scholar] [CrossRef]
- Mansouri Majd, S.; Salimi, A. Ultrasensitive flexible FET-type aptasensor for CA 125 cancer marker detection based on carboxylated multiwalled carbon nanotubes immobilized onto reduced graphene oxide film. Anal. Chim. Acta 2018, 1000, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Bao, J.; Zhao, Y.; Huo, D.; Chen, M.; Yang, M.; Fa, H.; Hou, C. A sensitive label-free electrochemical immunosensor for detection of cytokeratin 19 fragment antigen 21-1 based on 3D graphene with gold nanopaticle modified electrode. Talanta 2018, 178, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Amani, J.; Khoshroo, A.; Rahimi-Nasrabadi, M. Electrochemical immunosensor for the breast cancer marker CA 15-3 based on the catalytic activity of a CuS/reduced graphene oxide nanocomposite towards the electrooxidation of catechol. Microchim. Acta 2017, 185, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.A.; Sanchez, J.L.A.; O’Sullivan, C.K.; Abbas, M.N. DNA biosensors based on gold nanoparticles-modified graphene oxide for the detection of breast cancer biomarkers for early diagnosis. Bioelectrochemistry 2017, 118, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Liu, J.; Wang, J.; Zhao, H.; Ren, H.; Li, Z. Dual signal amplification strategy of Au nanopaticles/ZnO nanorods hybridized reduced graphene nanosheet and multienzyme functionalized Au@ZnO composites for ultrasensitive electrochemical detection of tumor biomarker. Biosens. Bioelectron. 2017, 97, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Khan, R. Graphene oxide layer decorated gold nanoparticles based immunosensor for the detection of prostate cancer risk factor. Anal. Biochem. 2017, 536, 51–58. [Google Scholar] [CrossRef]
- Yang, J.J.; Cao, J.T.; Wang, H.; Liu, Y.M.; Ren, S.W. Ferrocene-graphene sheets for high-efficiency quenching of electrochemiluminescence from Au nanoparticles functionalized cadmium sulfide flower-like three dimensional assemblies and sensitive detection of prostate specific antigen. Talanta 2017, 167, 325–332. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Bishop, G.W.; Bhakta, S.; El-Sawy, A.; Suib, S.L.; Rusling, J.F. Fe3O4 nanoparticles on graphene oxide sheets for isolation and ultrasensitive amperometric detection of cancer biomarker proteins. Biosens. Bioelectron. 2017, 91, 359–366. [Google Scholar] [CrossRef]
- He, L.; Pagneux, Q.; Larroulet, I.; Serrano, A.Y.; Pesquera, A.; Zurutuza, A.; Mandler, D.; Boukherroub, R.; Szunerits, S. Label-free femtomolar cancer biomarker detection in human serum using graphene-coated surface plasmon resonance chips. Biosens. Bioelectron. 2017, 89, 606–611. [Google Scholar] [CrossRef]
- Shahzad, F.; Zaidi, S.A.; Koo, C.M. Highly sensitive electrochemical sensor based on environmentally friendly biomass-derived sulfur-doped graphene for cancer biomarker detection. Sens. Actuators B Chem. 2017, 241, 716–724. [Google Scholar] [CrossRef]
- Amouzadeh Tabrizi, M.; Shamsipur, M.; Saber, R.; Sarkar, S. Simultaneous determination of CYC and VEGF165 tumor markers based on immobilization of flavin adenine dinucleotide and thionine as probes on reduced graphene oxide-poly (amidoamine)/gold nanocomposite modified dual working screen-printed electrode. Sens. Actuators B Chem. 2017, 240, 1174–1181. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wu, D.; Ma, H.; Pang, X.; Fan, D.; Wei, Q.; Du, B. Ultrasensitive label-free electrochemical immunosensor based on multifunctionalized graphene nanocomposites for the detection of alpha fetoprotein. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Singh, C.; Srivastava, S.; Admane, P.; Agrawal, V.V.; Sumana, G.; John, R.; Panda, A.; Dong, L.; Malhotra, B.D. Graphene oxide–metal nanocomposites for cancer biomarker detection. RSC Adv. 2017, 7, 35982–35991. [Google Scholar] [CrossRef] [Green Version]
- Miao, L.; Jiao, L.; Zhang, J.; Li, H. Amperometric sandwich immunoassay for the carcinoembryonic antigen using a glassy carbon electrode modified with iridium nanoparticles, polydopamine and reduced graphene oxide. Microchim. Acta 2017, 184, 169–175. [Google Scholar] [CrossRef]
- Pei, H.; Zhu, S.; Yang, M.; Kong, R.; Zheng, Y.; Qu, F. Graphene oxide quantum dots@silver core-shell nanocrystals as turn-on fluorescent nanoprobe for ultrasensitive detection of prostate specific antigen. Biosens. Bioelectron. 2015, 74, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, Y.; Yung, B.; Xiong, Y.; Chen, X. Nanotechnology-enhanced no-wash biosensors for in vitro diagnostics of cancer. ACS Nano 2017, 11, 5238–5292. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, S.R.; Lee, J.; Yeo, J.; Na, H.K.; Kim, Y.K.; Jang, H.; Lee, J.H.; Han, S.W.; Lee, Y.; Narry, K.V.; et al. Quantitative and multiplexed microRNA sensing in living cells based on peptide nucleic acid and nano graphene oxide (PANGO). J. Am. Chem. Soc. 2013, 7, 5882–5891. [Google Scholar] [CrossRef]
- Vilela, P.; El-Sagheer, A.; Millar, T.M.; Brown, T.; Muskens, O.L.; Kanaras, A.G. Graphene oxide-upconversion nanoparticle based optical sensors for targeted detection of mRNA biomarkers present in Alzheimer’s disease and prostate cancer. ACS Sens. 2017, 2, 52–56. [Google Scholar] [CrossRef]
- Duan, R.; Zhang, Z.; Zheng, F.; Wang, L.; Guo, J.; Zhang, T.; Dai, X.; Zhang, S.; Yang, D.; Kuang, R.; et al. Combining protein and miRNA quantification for bladder cancer analysis. ACS Appl. Mater. Interfaces 2017, 9, 23420–23427. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Lu, C.; Yang, X. Three kinds of DNA-directed nanoclusters cooperating with graphene oxide for assaying mucin 1, carcinoembryonic antigen and cancer antigen 125. Sens. Actuators B Chem. 2018, 262, 9–16. [Google Scholar]
- Jiang, H.; Li, F.R.; Li, W.; Lu, X.; Ling, K. Multiplexed determination of intracellular messenger RNA by using a graphene oxide nanoprobe modified with target-recognizing fluorescent oligonucleotides. Microchim. Acta 2018, 185, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhen, S.J.; Li, Y.F.; Huang, C.Z. Silver nanoparticles deposited on graphene oxide for ultrasensitive surface-enhanced Raman scattering immunoassay of cancer biomarker. Nanoscale 2018, 10, 11942–11947. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, H.; Huang, Z.; Li, T.; Deng, A.; Kong, J. DNase I enzyme-aided fluorescence signal amplification based on graphene oxide-DNA aptamer interactions for colorectal cancer exosome detection. Talanta 2018, 184, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, X.; Li, P.; Wang, Y.; Jiang, W. Terminal protection-mediated autocatalytic cascade amplification coupled with graphene oxide fluorescence switch for sensitive and rapid detection of folate receptor. Talanta 2017, 174, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, G.; Wu, P.; Cai, C. Real-time fluorescence assay of alkaline phosphatase in living cells using boron-doped graphene quantum dots as fluorophores. Biosens. Bioelectron. 2017, 96, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Kermani, H.A.; Hosseini, M.; Dadmehr, M.; Hosseinkhani, S.; Ganjali, M.R. DNA methyltransferase activity detection based on graphene quantum dots using fluorescence and fluorescence anisotropy. Sens. Actuators B Chem. 2017, 241, 217–223. [Google Scholar]
- Xu, X.; Wei, M.; Liu, Y.; Liu, X.; Wei, W.; Zhang, Y.; Liu, S. A simple, fast, label-free colorimetric method for detection of telomerase activity in urine by using hemin-graphene conjugates. Biosens. Bioelectron. 2017, 87, 600–606. [Google Scholar] [CrossRef]
- Zhang, H.; Ba, S.; Mahajan, D.; Lee, J.Y.; Ye, R.; Shao, F.; Lu, L.; Li, T. Versatile types of DNA-based nanobiosensors for specific detection of cancer biomarker FEN1 in living cells and cell-free systems. Nano Lett. 2018, 18, 7383–7388. [Google Scholar] [CrossRef]
- Kim, D.M.; Kim, D.H.; Jung, W.; Lee, K.Y.; Kim, D.E. Fluorometric detection of EGFR exon 19 deletion mutation in lung cancer cells using graphene oxide. Analyst 2018, 143, 1797–1804. [Google Scholar] [CrossRef]
- Xia, N.; Wang, X.; Liu, L. A graphene oxide-based fluorescent method for the detection of human chorionic gonadotropin. Sensors 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Meng, H.M.; Zhao, D.; Li, N.; Chang, J. A graphene quantum dot-based multifunctional two-photon nanoprobe for the detection and imaging of intracellular glutathione and enhanced photodynamic therapy. Analyst 2018, 143, 4967–4973. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Zhan, S.; Sun, C.; Cheng, Y.; Wang, X.; Liu, B.; Zhai, T.; Lou, X.; Xia, F. Simultaneous detection of telomerase and miRNA with graphene oxide-based fluorescent aptasensor in living cells and tissue samples. Biosens. Bioelectron. 2019, 124–125, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, X.; Wu, F.G. A graphene oxide-based switch-on fluorescent probe for glutathione detection and cancer diagnosis. J. Colloid Interface Sci. 2018, 530, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shi, M.; Huang, H.; Hu, K.; Chen, W.; Huang, Y.; Zhao, S. A fluorescent aptasensor based on single oligonucleotide-mediated isothermal quadratic amplification and graphene oxide fluorescence quenching for ultrasensitive protein detection. Analyst 2018, 143, 3918–3925. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yao, Y.; Wu, J.; Cui, L.; Zhang, Y.; Geng, D.; Tao, D.; Yu, Y. A robust fluorescent probe for detection of telomerase activity in vitro and imaging in living cells via telomerase-triggering primer extension to desorb DNA from graphene oxide. Analyst 2018, 143, 3651–3660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Peng, J.; Hong, M.F.; Chen, J.Q.; Liang, R.P.; Qiu, J.D. A facile graphene oxide-based fluorescent nanosensor for the in situ “turn-on” detection of telomerase activity. Analyst 2018, 143, 2334–2341. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Tan, H.; Sun, Y.; Wang, J.; Hang, Y.; Lu, N.; Yang, J.; Qu, X.; Hua, J. Graphene oxide-based NIR fluorescence probe with aggregation-induced emission property for lectins detection and liver cells targeting. Sens. Actuators B Chem. 2018, 261, 115–126. [Google Scholar] [CrossRef]
- Mitterhauser, M.; Wadsak, W. Imaging biomarkers or biomarker imaging? Pharmaceuticals 2014, 7, 765–778. [Google Scholar] [CrossRef]
- Fan, Z.; Li, Y.; Li, X.; Fan, L.; Zhou, S.; Fang, D.; Yang, S. Surrounding media sensitive photoluminescence of boron-doped graphene quantum dots for highly fluorescent dyed crystals, chemical sensing and bioimaging. Carbon 2014, 70, 149–156. [Google Scholar] [CrossRef]
- Liu, F.; Gao, Y.; Li, H.; Sun, S. Interaction of propidium iodide with graphene oxide and its application for live cell staining. Carbon 2014, 71, 190–195. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, C.; Zheng, X.; Gao, L.; Cui, Z.; Yang, H.; Guo, C.; Chi, Y.; Li, C.M. One-step and high yield simultaneous preparation of single- and multi-layer graphene quantum dots from CX-72 carbon black. J. Mater. Chem. 2012, 22, 8764. [Google Scholar] [CrossRef]
- Hu, C.; Liu, Y.; Yang, Y.; Cui, J.; Huang, Z.; Wang, Y.; Yang, L.; Wang, H.; Xiao, Y.; Rong, J. One-step preparation of nitrogen-doped graphene quantum dots from oxidized debris of graphene oxide. J. Mater. Chem. B 2013, 1, 39–42. [Google Scholar] [CrossRef]
- Maji, S.K.; Mandal, A.K.; Nguyen, K.T.; Borah, P.; Zhao, Y. Cancer cell detection and therapeutics using peroxidase-active nanohybrid of gold nanoparticle-loaded mesoporous silica-coated graphene. ACS Appl. Mater. Interfaces 2015, 7, 9807–9816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cao, Y.; Chong, Y.; Ma, Y.; Zhang, H.; Deng, Z.; Hu, C.; Zhang, Z. Graphene oxide based theranostic platform for T1-weighted magnetic resonance imaging and drug delivery. ACS Appl. Mater. Interfaces 2013, 5, 13325–13332. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xiao, X.; Zuo, X.; Liang, Y.; Nan, J. Hydrothermal preparation of photoluminescent graphene quantum dots characterized excitation-independent emission and its application as a bioimaging reagent. Part. Part. Syst. Character 2014, 31, 801–809. [Google Scholar] [CrossRef]
- S un, H.; Wu, L.; Gao, N.; Ren, J.; Qu, X. Improvement of photoluminescence of graphene quantum dots with a biocompatible photochemical reduction pathway and its bioimaging application. ACS Appl. Mater. Interfaces 2013, 5, 1174–1179. [Google Scholar] [CrossRef]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene quantum dots derived from carbon fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef]
- Shi, J.; Lyu, J.; Tian, F.; Yang, M. A fluorescence turn-on biosensor based on graphene quantum dots (GQDs) and molybdenum disulfide (MoS2) nanosheets for epithelial cell adhesion molecule (EpCAM) detection. Biosens. Bioelectron. 2017, 93, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bao, C.; Liang, S.; Fu, H.; Wang, K.; Deng, M.; Liao, Q.; Cui, D. RGD-conjugated silica-coated gold nanorods on the surface of carbon nanotubes for targeted photoacoustic imaging of gastric cancer. Nanoscale Res. Lett. 2014, 9, 264. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Singh, V.; Umrao, S.; Parashar, V.; Abraham, S.; Singh, A.K.; Nath, G.; Saxena, P.S.; Srivastava, A. Facile, rapid and upscaled synthesis of green luminescent functional graphene quantum dots for bioimaging. RSC Adv. 2014, 4, 21101. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, Y.; He, N.; Zhang, Y.; Lu, Z.; Zhang, J.; Zhang, Z. Preparation of graphene quantum dots for bioimaging application. J. Nanosci. Nanotechnol. 2012, 12, 2924–2928. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, K.; Sun, L.; Sun, B.; Yang, M.; Chen, H.; Wang, Y.; Sun, J.; Dong, L. Application of graphene quantum dots for simultaneous fluorescence imaging and tumor-targeted drug delivery. Sens. Actuators B Chem. 2018, 256, 616–623. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, J.T.; Tabakman, S.M.; Liang, Y.; Wang, H.; Casalongue, H.S.; Vinh, D.; Dai, H. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831. [Google Scholar] [CrossRef]
- Huang, R.C.; Chiu, W.J.; Po-Jung Lai, I.; Huang, C.C. Multivalent aptamer/gold nanoparticle-modified graphene oxide for mass spectrometry-based tumor tissue imaging. Sci. Rep. 2015, 5, 10292. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tseng, Y.T.; Suo, G.; Chen, L.; Yu, J.; Chiu, W.J.; Huang, C.C.; Lin, C.H. Photothermal therapeutic response of cancer cells to aptamer-gold nanoparticle-hybridized graphene oxide under NIR illumination. ACS Appl. Mater. Interfaces 2015, 7, 5097–5106. [Google Scholar] [CrossRef]
- Feng, D.; Song, Y.; Shi, W.; Li, X.; Ma, H. Distinguishing folate-receptor-positive cells from folate-receptor-negative cells using a fluorescence off-on nanoprobe. Anal. Chem. 2013, 85, 6530–6535. [Google Scholar] [CrossRef]
- Hu, C.; Liu, Y.; Qin, J.; Nie, G.; Lei, B.; Xiao, Y.; Zheng, M.; Rong, J. Fabrication of reduced graphene oxide and sliver nanoparticle hybrids for Raman detection of absorbed folic acid: A potential cancer diagnostic probe. ACS Appl. Mater. Interfaces 2013, 5, 4760–6768. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.L.; Huang, C.C.; Mai, F.-D.; Yen, C.L.; Tzing, S.H.; Hsieh, H.T.; Ling, Y.C.; Chang, J.Y. Application of paramagnetic graphene quantum dots as a platform for simultaneous dual-modality bioimaging and tumor-targeted drug delivery. J. Mater. Chem. B 2015, 3, 651–664. [Google Scholar] [CrossRef]
- Nergiz, S.Z.; Gandra, N.; Tadepalli, S.; Singamaneni, S. Multifunctional hybrid nanopatches of graphene oxide and gold nanostars for ultraefficient photothermal cancer therapy. ACS Appl. Mater. Interfaces 2014, 6, 16395–16402. [Google Scholar] [CrossRef] [PubMed]
- Gui, W.; Zhang, J.; Chen, X.; Yu, D.; Ma, Q. N-Doped graphene quantum dot@mesoporous silica nanoparticles modified with hyaluronic acid for fluorescent imaging of tumor cells and drug delivery. Mikrochim. Acta 2017, 185, 66. [Google Scholar] [CrossRef] [PubMed]
- Nigam, P.; Waghmode, S.; Louis, M.; Wangnoo, S.; Chavan, P.; Sarkar, D. Graphene quantum dots conjugated albumin nanoparticles for targeted drug delivery and imaging of pancreatic cancer. J. Mater. Chem. B 2014, 2, 3190–3195. [Google Scholar] [CrossRef]
- Kim, K.S.; Hur, W.; Park, S.-J.; Hong, S.W.; Choi, J.E.; Goh, E.J.; Yoon, S.K.; Hahn, S.K. Bioimaging for targeted delivery of hyaluronic acid derivatives to the livers in cirrhotic mice using quantum dots. ACS Nano 2010, 4, 3005–3014. [Google Scholar] [CrossRef]
- Jung, H.S.; Kong, W.H.; Sung, D.K.; Lee, M.-Y.; Beack, S.E.; Keum, D.H.; Kim, K.S.; Yun, S.H.; Hahn, S.K. Nanographene oxide–hyaluronic acid conjugate for photothermal ablation therapy of skin cancer. ACS Nano 2014, 8, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; He, Y.J.; Chen, X.W.; Wang, J.H. Quantum-dot-conjugated graphene as a probe for simultaneous cancer-targeted fluorescent imaging, tracking, and monitoring drug delivery. Bioconjug. Chem. 2013, 24, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Shi, H.; Wu, H.; Zhang, Y.; Wang, X.; Wu, D.; An, L.; Yang, S. Prostate cancer targeted multifunctionalized graphene oxide for magnetic resonance imaging and drug delivery. Carbon 2016, 107, 87–99. [Google Scholar] [CrossRef]
- Zheng, X.T.; He, H.L.; Li, C.M. Multifunctional graphene quantum dots-conjugated titanate nanoflowers for fluorescence-trackable targeted drug delivery. RSC Adv. 2013, 3, 24853. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q.; Chu, X.; Chen, T.; Ge, J.; Yu, R. Graphene oxide-peptide conjugate as an intracellular protease sensor for caspase-3 activation imaging in live cells. Angew. Chem. Int. Ed. 2011, 50, 7065–7069. [Google Scholar] [CrossRef]
- Su, Z.; Shen, H.; Wang, H.; Wang, J.; Li, J.; Nienhaus, G.U.; Shang, L.; Wei, G. Motif-designed peptide nanofibers decorated with graphene quantum dots for simultaneous targeting and imaging of tumor cells. Adv. Funct. Mater. 2015, 25, 5472–5478. [Google Scholar] [CrossRef]
- Dong, H.; Dai, W.; Ju, H.; Lu, H.; Wang, S.; Xu, L.; Zhou, S.F.; Zhang, Y.; Zhang, X. Multifunctional poly(L-lactide)-polyethylene glycol-grafted graphene quantum dots for intracellular microRNA imaging and combined specific-gene-targeting agents delivery for improved therapeutics. ACS Appl. Mater. Interfaces 2015, 7, 11015–11023. [Google Scholar] [CrossRef]
- Li, L.; Feng, J.; Liu, H.; Li, Q.; Tong, L.; Tang, B. Two-color imaging of microRNA with enzyme-free signal amplification via hybridization chain reactions in living cells. Chem. Sci. 2016, 7, 1940–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Liu, C.; Ren, W.; Li, Z. Graphene surface-anchored fluorescence sensor for sensitive detection of microRNA coupled with enzyme-free signal amplification of hybridization chain reaction. ACS Appl. Mater. Interfaces 2012, 4, 6450–6453. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T.; Cao, L.; Pengju, G.L.; Lu, F.; Wang, X.; Wang, H.; Mohammed, J.M.; Liu, Y.; Qi, G.; Sun, Y.P. Carbon dots for optical imaging in vivo. J. Am. Chem. Soc. 2009, 131, 11308–11309. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.T.; Liu, Z. Graphene in mice: Ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef] [PubMed]
- Abdullah Al, N.; Lee, J.E.; In, I.; Lee, H.; Lee, K.D.; Jeong, J.H.; Park, S.Y. Target delivery and cell imaging using hyaluronic acid-functionalized graphene quantum dots. Mol. Pharm. 2013, 10, 3736–3744. [Google Scholar] [CrossRef] [PubMed]
- Nahain, A.A.; Lee, J.E.; Jeong, J.H.; Park, S.Y. Photoresponsive fluorescent reduced graphene oxide by spiropyran conjugated hyaluronic acid for in vivo imaging and target delivery. Biomacromolecules 2013, 14, 4082–4090. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, B.; Wang, M.; Li, J.; Pan, W.; Gao, X.; Li, N.; Tang, B. A highly sensitive strategy for fluorescence imaging of microRNA in living cells and in vivo based on graphene oxide-enhanced signal molecules quenching of molecular beacon. ACS Appl. Mater. Interfaces 2018, 10, 6982–6990. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, A.; Qin, M.; Huang, R.; Zhang, G.; Xue, B.; Wei, J.; Li, Y.; Cao, Y.; Wang, W. Hierarchical construction of a mechanically stable peptide-graphene oxide hybrid hydrogel for drug delivery and pulsatile triggered release in vivo. Nanoscale 2015, 7, 1655–1660. [Google Scholar] [CrossRef]
- Cornelissen, B.; Able, S.; Kersemans, V.; Waghorn, P.A.; Myhra, S.; Jurkshat, K.; Crossley, A.; Vallis, K.A. Nanographene oxide-based radioimmunoconstructs for in vivo targeting and SPECT imaging of HER2-positive tumors. Biomaterials 2013, 34, 1146–1154. [Google Scholar] [CrossRef]
- Kalluru, P.; Vankayala, R.; Chiang, C.S.; Hwang, K.C. Nano-graphene oxide-mediated in vivo fluorescence imaging and bimodal photodynamic and photothermal destruction of tumors. Biomaterials 2016, 95, 1–10. [Google Scholar] [CrossRef]
- Dawidczyk, C.M.; Russell, L.M.; Searson, P.C. Nanomedicines for cancer therapy: State-of-the-art and limitations to pre-clinical studies that hinder future developments. Front. Chem. 2014, 2, 69. [Google Scholar] [CrossRef] [PubMed]










| Material | Target biomarker | Detection limit | Ref. |
|---|---|---|---|
| CdS nanocrystals/graphene oxide-AuNPs | p53 | 4 fg/ml | [86] |
| AgNPs in PANI and N-doped graphene | miRNA-21 | 0.2 fM | [87] |
| 3D porous network graphene aerogel | CA 153 | 0.03 mU/mL | [88] |
| ss-DNA amino-functionalized GQD | mRNA-25 | 95.0 pM | [89] |
| AuPtPd/rGO | H2O2 | 2 nM | [90] |
| rGO/ordered mesoporous carbon/Ni-oxytetracycline metallopolymer NPs | EGFR exon21 L858R point mutation | 120 nM | [91] |
| AFP aptamer GO | AFP | 3 pg/mL | [92] |
| CysA/Au NSs/GQDs | CA 153 | 0.11 U/mL | [93] |
| GO-COOH | cytokeratin 19 | 1 fg/mL | [94] |
| Cubic CeO2/RGO | Cyfra-21-1 | 0.625 pg/mL | [95] |
| Anti-CEA/PBSE/Graphene/Cu | CEA | 0.23 ng/mL | [96] |
| Ab1/P5FIn/erGO, Ab2/GQDs@AuNP | CEA | 3.78 fg/mL | [97] |
| GS-PS67-b-PAA27-Au | PSA | 40 fg/mL | [98] |
| Hemin-GS/PdNPs | PSA | 8 pg/mL | [99] |
| Pd@Au@Pt/COOH-rGO | PSACEA | 8 pg/mL 2 pg/mL | [100] |
| MWCNTs-COOH/rGO | CA 125 | 0.5 nU/mL | [101] |
| ss-DNA/3D GF/Ag NPs | CYFRA21-1 | 10 fM | [60] |
| anti-CYFRA21-1/3D graphene@Au NPs | CYFRA 21-1 | 100 pg/mL | [102] |
| CuS/rGO | CA 153 | 0.3 U/mL | [103] |
| ERBB2c, CD24c modified Au NPs/GO | HER2 | 0.16 nM, 0.23 nM | [104] |
| Au/ZnO/RGO | AFP | 0.01 pg/mL | [105] |
| anti-PSA/Au NPs/GO | PSA | 0.24 fg/mL | [106] |
| Au–S-GS | CEA NMP22 | 25 fg/mL 30 fg/mL | [68] |
| Fc-GNs/aptamer/BSA/DNA/Au-CdS flower-like 3D assemblies | PSA | 0.38 pg/mL | [107] |
| rGO/Fe3O4@GO | PSA PS membrane antigen | 15 fg/mL 4.8 fg/mL | [108] |
| graphene coated SPR chip | FAP | 5 fM | [109] |
| sulfur-doped rGO | 8-hydroxy-2’-deoxyguanosine | 1 nM | [110] |
| FAD/Th/rGO-PAMAM/Aunano | CYC VEGF165 | 63.9 pM 38.4 pM | [111] |
| TB-Au-Fe3O4-rGO | AFP | 2.7 fg/mL | [112] |
| rGO–metal nanocomposites | ErbB2 | <1 fM | [113] |
| Anti-CEA/PDA-rGO | CEA | 0.23 pg/mL | [114] |
| Probe | Target biomarker | Detection limit | Ref. |
|---|---|---|---|
| Aptamer-GO probe | C-myc/TK1/actin | 0.26 nM | [121] |
| Ag NPs/GO | PSA | 0.23 pg/mL | [122] |
| DNA aptamer GO | Exosome | 21000 particles/μl | [123] |
| PT-DNA/GO | folate receptor | 0.44 pM | [124] |
| boron-doped GQD | alkaline phosphatase | 10±5 cells/mL | [125] |
| ssDNA/GQD | CYFRA21-1 | 0.3 μU/mL | [126] |
| Hemin-GS | Telomerase | 60 cells/mL | [127] |
| DNA-GO nanocomposites | Flap structure-specific endonuclease 1 | 0.38 pM | [128] |
| DNA/GO | epidermal growth factor receptor | 390 pg | [129] |
| DNA/GO | hCG | 20 mIU/mL | [130] |
| GQD@MnO2 | glutathione | 83 nM | [131] |
| telomerase/miR-21 oligonucleotides/GO | Telomerase/mRNA 21 | 10 pM/10 HeLa cells | [132] |
| GO/MnO2/fluorescein | glutathione | 1.53 μM | [133] |
| DNA/GO | CEA | 28.5 fg/mL | [134] |
| DNA/GO | telomerase | 2.7 cells | [135] |
| DNA/GO | telomerase | 30 cells | [136] |
| PT-Man@GO, PT-Gal@GO | lectin | 7.9 nM | [137] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, H.; Tang, H.; Xiong, P.; Zhou, Z. Biomarkers-based Biosensing and Bioimaging with Graphene for Cancer Diagnosis. Nanomaterials 2019, 9, 130. https://doi.org/10.3390/nano9010130
Gu H, Tang H, Xiong P, Zhou Z. Biomarkers-based Biosensing and Bioimaging with Graphene for Cancer Diagnosis. Nanomaterials. 2019; 9(1):130. https://doi.org/10.3390/nano9010130
Chicago/Turabian StyleGu, Hui, Huiling Tang, Ping Xiong, and Zhihua Zhou. 2019. "Biomarkers-based Biosensing and Bioimaging with Graphene for Cancer Diagnosis" Nanomaterials 9, no. 1: 130. https://doi.org/10.3390/nano9010130
APA StyleGu, H., Tang, H., Xiong, P., & Zhou, Z. (2019). Biomarkers-based Biosensing and Bioimaging with Graphene for Cancer Diagnosis. Nanomaterials, 9(1), 130. https://doi.org/10.3390/nano9010130