Magnetite-Supported Gold Nanostars for the Uptake and SERS Detection of Tetracycline
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of MNP-AuNS
2.2. Uptake of TC from Aqueous Solutions Using MNP-AuNS Nanosorbents
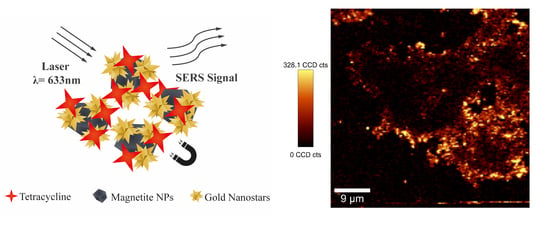
2.3. SERS Detection of Tetracycline Using MNP-AuNS Substrates
2.4. SERS Detection of Tetracycline in More Complex Spiked Aqueous Matrices
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Fe3O4 Nanoparticles
3.3. Synthesis of MNP-Au Seeds
3.4. Synthesis of MNP-AuNS Nanocomposite
3.5. Adsorption Experiments of TC onto MNP-AuNS
3.6. SERS Measurements
3.7. Instrumentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Boxall, A.B.A.; Kolpin, D.W.; Halling-Sørensen, B.; Tolls, J. Are veterinary medicines causing environmental risks? Environ. Sci. Technol. 2003, 37, 286A–294A. [Google Scholar] [CrossRef] [PubMed]
- Granados-Chinchilla, F.; Rodríguez, C. Tetracyclines in food and feedingstuffs: From regulation to analytical methods, bacterial resistance, and environmental and health implications. J. Anal. Methods Chem. 2017, 2017, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Zhang, L.; Lei, J.; Zong, L.; Li, J.; Wu, Z.; Wang, Z. Overview of emerging contaminants and associated human health effects. BioMed Res. Int. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.L.; Levy, S.B. The history of the tetracyclines. Ann. N. Y. Acad. Sci. 2011, 1241, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Duggar, B.M. Aureomycin: A product of the continuing search for new antibiotics. Ann. N. Y. Acad. Sci. 1948, 51, 177–181. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Sales of Veterinary Antimicrobial Agents in 30 European Countries in 2015. Available online: https://www.ema.europa.eu/documents/report/seventh-esvac-report-sales-veterinary-antimicrobial-agents-30-european-countries-2015_en.pdf (accessed on 14 November 2018).
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Bottoni, P.; Caroli, S. Presence of residues and metabolites of pharmaceuticals in environmental compartments, food commodities and workplaces: A review spanning the three-year period 2014-2016. Microchem. J. 2018, 136, 2–24. [Google Scholar] [CrossRef]
- Commission, E. No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Union 2010, L15, 1–72. [Google Scholar]
- Kowalski, P. Capillary electrophoretic method for the simultaneous determination of tetracycline residues in fish samples. J. Pharm. Biomed. Anal. 2008, 47, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Croubels, S.; Van Peteghem, C.; Baeyens, W. Sensitive spectrofluorimetric determination of tetracycline residues in bovine milk. Analyst 1994, 119, 2713–2716. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.W.; Zuo, Y. Simultaneous determination of tetracycline, oxytetracycline, and 4-epitetracycline in milk by high-performance liquid chromatography. Food Chem. 2007, 105, 1297–1301. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, S.; Liu, W.; Zhao, C.; Xi, R. Preparation of anti-tetracycline antibodies and development of an indirect heterologous competitive enzyme-linked immunosorbent assay to detect residues of tetracycline in milk. J. Agric. Food Chem. 2007, 55, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Yu, M.; Wang, Y.; Hu, W.; Cheng, D.; Swihart, M.T.; Song, Y. Multi-color quantum dot-based fluorescence immunoassay array for simultaneous visual detection of multiple antibiotic residues in milk. Biosens. Bioelectron. 2015, 72, 320–325. [Google Scholar] [CrossRef]
- Conzuelo, F.; Campuzano, S.; Gamella, M.; Pinacho, D.G.; Reviejo, A.J.; Marco, M.P.; Pingarrón, J.M. Integrated disposable electrochemical immunosensors for the simultaneous determination of sulfonamide and tetracycline antibiotics residues in milk. Biosens. Bioelectron. 2013, 50, 100–105. [Google Scholar] [CrossRef]
- Dang, P.K.; Degand, G.; Danyi, S.; Pierret, G.; Delahaut, P.; Ton, V.D.; Maghuin-Rogister, G.; Scippo, M.-L. Validation of a two-plate microbiological method for screening antibiotic residues in shrimp tissue. Anal. Chim. Acta 2010, 672, 30–39. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-enhanced Raman spectroscopy: A brief retrospective. J. Raman Spectrosc. 2005, 36, 485–496. [Google Scholar] [CrossRef]
- Kneipp, H.; Kneipp, K. Surface-enhanced hyper Raman scattering in silver colloidal solutions. J. Raman Spectrosc. 2005, 36, 551–554. [Google Scholar] [CrossRef]
- Kneipp, K. Surface-enhanced raman scattering. Phys. Today 2007, 60, 40–46. [Google Scholar] [CrossRef]
- Fateixa, S.; Nogueira, H.I.S.; Trindade, T. Hybrid nanostructures for SERS: Materials development and chemical detection. Phys. Chem. Chem. Phys. 2015, 17, 21046–21071. [Google Scholar] [CrossRef]
- Schlücker, S. Surface-enhanced raman spectroscopy: Concepts and chemical applications. Angew. Chem. 2014, 53, 4756–4795. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, Q.; Hassan, M.M.; Chen, X.; Ouyang, Q.; Guo, Z.; Zhao, J. A magnetite/PMAA nanospheres-targeting SERS aptasensor for tetracycline sensing using mercapto molecules embedded core/shell nanoparticles for signal amplification. Biosens. Bioelectron. 2017, 92, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, P.; Yuan, H.; Peng, Y.; Hong, Q.; Liu, M. Rapid detection of tetracycline residues in duck meat using surface enhanced raman spectroscopy. J. Spectrosc. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Qu, L.-L.; Liu, Y.-Y.; Liu, M.; Yang, G.-H.; Li, D.-W.; Li, H. Highly reproducible Ag NPs/CNT-intercalated GO membranes for enrichment and SERS detection of antibiotics. ACS Appl. Mater. Interfaces 2016, 8, 28180–28186. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Ma, X.; Duan, N.; Wu, S.; Xia, Y.; Wang, Z.; Xu, B. Ultrasensitive SERS aptasensor for the detection of oxytetracycline based on a gold-enhanced nano-assembly. Talanta 2017, 165, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S.; Chao, K.; Huang, Q.; Kim, M.; Schmidt, W.; Qin, J.; Broadhurst, C.L. A simple surface-enhanced Raman spectroscopic method for on-site screening of tetracycline residue in whole milk. Sensors 2018, 18, 424. [Google Scholar] [CrossRef]
- Filgueiras, A.L.; Paschoal, D.; Dos Santos, H.F.; Sant’Ana, A.C. Adsorption study of antibiotics on silver nanoparticle surfaces by surface-enhanced Raman scattering spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Bai, Y.; Chen, H.; Liu, S.; Chen, N.; Huang, J.; Huang, S.; Chen, Z. SERS detection of expired tetracycline hydrochloride with an optical fiber nano-probe. Anal. Methods 2015, 7, 1307–1312. [Google Scholar] [CrossRef]
- Lee, S.; Kumar, P.; Hu, Y.; Cheng, G.J.; Irudayaraj, J. Graphene laminated gold bipyramids as sensitive detection platforms for antibiotic molecules. Chem. Commun. 2015, 51, 15494–15497. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Zhang, H.; Chen, Q.-W.; Yan, N.; Wang, H. Improved surface-enhanced Raman scattering on micro-scale Au hollow spheres: Synthesis and application in detecting tetracycline. Analyst 2011, 136, 2527–2532. [Google Scholar] [CrossRef]
- Pinheiro, P.C.; Daniel-da-Silva, A.L.; Nogueira, H.I.S.; Trindade, T. Functionalized inorganic nanoparticles for magnetic separation and SERS detection of water pollutants. Eur. J. Inorg. Chem. 2018, 2018, 3443–3461. [Google Scholar] [CrossRef]
- Pinheiro, P.C.; Fateixa, S.; Trindade, T. SERS detection of penicillin G using magnetite decorated with gold nanoparticles. Magnetochemistry 2017, 3, 32. [Google Scholar] [CrossRef]
- Reguera, J.; Aberasturi, D.J.; Winckelmans, N.; Langer, J.; Bals, S.; Liz-Marzán, L.M. Synthesis of Janus plasmonic-magnetic, star-sphere nanoparticles, and their application in SERS detection. Faraday Discuss. 2016, 191, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Neng, J.; Tan, J.; Jia, K.; Sun, P. A fast and cost-effective detection of melamine by surface enhanced Raman spectroscopy using a novel hydrogen bonding-assisted supramolecular matrix and gold-coated magnetic nanoparticles. Appl. Sci. 2017, 7, 475. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, J.; Mi, L.; Gong, H.; Jiang, S.; Yu, Q. Multifunctional magnetic-plasmonic nanoparticles for fast concentration and sensitive detection of bacteria using SERS. Biosens. Bioelectron. Bioelectron. 2012, 31, 130–136. [Google Scholar] [CrossRef]
- Fan, Z.; Senapati, D.; Khan, A.S.; Singh, A.K.; Hamme, A.; Yust, B.; Sardar, D.; Ray, P.C. Popcorn-shaped magnetic core-plasmonic shell multifunctional nanoparticles for the targeted magnetic separation and enrichment, label-free SERS imaging, and photothermal destruction of multidrug-resistant bacteria. Chem. Eur. J. 2013, 19, 2839–2847. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhao, A.; Li, L.; Sun, H.; Wang, D.; Guo, H.; Sun, M.; Chen, P. Fabrication of Fe3O4@SiO2@Ag magnetic—Plasmonic nanospindles as highly efficient SERS active substrates for label-free detection of pesticides. New J. Chem. 2017, 41, 1582–1590. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.; Wang, C.; Rong, Z.; Ding, H.; Li, H.; Li, S.; Shao, N.; Dong, P.; Xiao, R.; et al. Facile synthesis of Au-coated magnetic nanoparticles and their application in bacteria detection via a SERS method. ACS Appl. Mater. Interfaces 2016, 8, 19958–19967. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Wang, J.; Rong, Z.; Li, P.; Xiao, R.; Wang, S. Polyethylenimine-interlayered silver-shell magnetic-core microspheres as multifunctional SERS substrates. J. Mater. Chem. C 2015, 3, 8684–8693. [Google Scholar] [CrossRef]
- Yu, W.; Huang, Y.; Pei, L.; Fan, Y.; Wang, X.; Lai, K. Magnetic Fe3O4/Ag hybrid nanoparticles as surface-enhanced raman scattering substrate for trace analysis of furazolidone in fish feeds. J. Nanomater. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Martin, F.L.; Zhang, D. Diagnose pathogens in drinking water via magnetic surface-enhanced Raman scattering (SERS) assay. Mater. Today Proc. 2017, 4, 25–31. [Google Scholar] [CrossRef]
- Du, J.; Jing, C. Preparation of thiol modified Fe3O4@Ag magnetic SERS probe for PAHs detection and identification. J. Phys. Chem. C 2011, 115, 17829–17835. [Google Scholar] [CrossRef]
- Kibar, G.; Topal, A.E.; Dana, A.; Tuncel, A. Newly designed silver coated-magnetic, monodisperse polymeric microbeads as SERS substrate for low-level detection of amoxicillin. J. Mol. Struct. 2016, 1119, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Li, Y.; Huang, H.; Li, P.; Guo, Z.; Luo, Q.; Wang, Z.; Chu, P.K.; Li, J.; Yu, X.-F. Efficient enrichment and self-assembly of hybrid nanoparticles into removable and magnetic SERS substrates for sensitive detection of environmental pollutants. ACS Appl. Mater. Interfaces 2017, 9, 7472–7480. [Google Scholar] [CrossRef]
- Hou, T.; Liu, Y.; Xu, L.; Wu, Y.; Ying, Y.; Wen, Y.; Guo, X.; Yang, H. Au dotted magnetic graphene sheets for sensitive detection of thiocyanate. Sens. Actuators B Chem. 2017, 241, 376–382. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, L.; Zhang, F.; Song, Z.; Hu, Y.; Ji, Y.; Shen, J.; Li, B.; Lu, H.; Yang, H. A promising magnetic SERS immunosensor for sensitive detection of avian influenza virus. Biosens. Bioelectron. 2017, 89, 906–912. [Google Scholar] [CrossRef]
- Tavares, D.S.; Daniel-da-Silva, A.L.; Lopes, C.B.; Silva, N.J.O.; Amaral, V.S.; Rocha, J.; Pereira, E.; Trindade, T. Efficient sorbents based on magnetite coated with siliceous hybrid shells for removal of mercury ions. J. Mater. Chem. A 2013, 1, 8134–8143. [Google Scholar] [CrossRef]
- Girginova, P.I.; Daniel-da-Silva, A.L.; Lopes, C.B.; Figueira, P.; Otero, M.; Amaral, V.S.; Pereira, E.; Trindade, T. Silica coated magnetite particles for magnetic removal of Hg(2+) from water. J. Colloid Interface Sci. 2010, 345, 234–240. [Google Scholar] [CrossRef]
- Wang, Y.; Polavarapu, L.; Liz-Marzán, L.M. Reduced graphene oxide-supported gold nanostars for improved SERS sensing and drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 21798–21805. [Google Scholar] [CrossRef]
- Sau, T.K.; Rogach, A.L.; Döblinger, M.; Feldmann, J. One-step high-yield aqueous synthesis of size-tunable multispiked gold nanoparticles. Small 2011, 7, 2188–2194. [Google Scholar] [CrossRef]
- Liu, M.; Guyot-Sionnest, P. Mechanism of silver(I)-assisted growth of gold nanorods and bipyramids. J. Phys. Chem. B 2005, 109, 22192–22200. [Google Scholar] [CrossRef] [PubMed]
- Orendorff, C.J.; Murphy, C.J. Quantitation of metal content in the silver-assisted growth of gold nanorods. J. Phys. Chem. B 2006, 110, 3990–3994. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, P.; Osório, I.; Dória, G.; Carvalho, P.A.; Pereira, A.; Langer, J.; Araújo, J.P.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Franco, R.; et al. Star-shaped magnetite@gold nanoparticles for protein magnetic separation and SERS detection. RSC Adv. 2014, 4, 3659–3667. [Google Scholar] [CrossRef]
- Leypold, C.F.; Reiher, M.; Brehm, G.; Schmitt, M.O.; Schneider, S.; Matousek, P.; Towrie, M. Tetracycline and derivatives-assignment of IR and Raman spectra via DFT calculations. Phys. Chem. Chem. Phys. 2003, 5, 1149–1157. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Karthikeyan, K.G. Interaction of tetracycline with aluminum and iron hydrous oxides. Environ. Sci. Technol. 2005, 39, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, R.A.; Mackay, A.A. Sorption of oxytetracycline to iron oxides and iron oxide-rich soils. Environ. Sci. Technol. 2005, 39, 6664–6671. [Google Scholar] [CrossRef]
- Zhang, D.; Niu, H.; Zhang, X.; Meng, Z.; Cai, Y. Strong adsorption of chlorotetracycline on magnetite nanoparticles. J. Hazard. Mater. 2011, 192, 1088–1093. [Google Scholar] [CrossRef]
- Zhao, Y.; Geng, J.; Wang, X.; Gu, X.; Gao, S. Adsorption of tetracycline onto goethite in the presence of metal cations and humic substances. J. Colloid Interface Sci. 2011, 361, 247–251. [Google Scholar] [CrossRef]
- Rakshit, S.; Sarkar, D.; Elzinga, E.J.; Punamiya, P.; Datta, R. Surface complexation of oxytetracycline by magnetite: Effect of solution properties. Vadose Zone J. 2014, 13, 1–10. [Google Scholar] [CrossRef]
- Rakshit, S.; Sarkar, D.; Punamiya, P.; Datta, R. Kinetics of oxytetracycline sorption on magnetite nanoparticles. Int. J. Environ. Sci. Technol. 2014, 11, 1207–1214. [Google Scholar] [CrossRef]
- Kuttner, C. Plasmonics in Sensing: From Colorimetry to SERS Analytics. In Plasmonics; Gric, T., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Sivapalan, S.T.; DeVetter, B.M.; Yang, T.K.; van Dijk, T.; Schulmerich, M.V.; Carney, P.S.; Rohit Bhargava, R.; Murphy, C.J. Off-resonance surface-enhanced Raman spectroscopy from gold nanorod suspensions as a function of aspect ratio: Not what we thought. ACS Nano 2013, 7, 2099–2105. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lorenzo, L.; Álvarez-Puebla, R.N.A.; Pastoriza-Santos, I.; Mazzucco, S.; Stéphan, O.; Kociak, M.; Liz-Marzán, L.M.; García de Abajo, F.J. Zeptomol Detection Through Controlled Ultrasensitive Surface-Enhanced Raman Scattering. J. Am. Chem. Soc. 2009, 131, 4616–4618. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Liang, W.; Yang, C.; Lin, W.; Bi, M. Simultaneous quantitative detection of tetracyclines derivatives by Raman spectroscopy. In Proceedings of the 2012 IEEE International Conference on Virtual Environments Human-Computer Interfaces and Measurement Systems (VECIMS) Proceedings, Tianjin, China, 2–4 July 2012; pp. 111–114. [Google Scholar] [CrossRef]
- Osawa, M.; Matsuda, N.; Yoshii, K.; Uchida, I. Charge transfer resonance Raman process in surface-enhanced Raman-scattering from p-aminothiophenol adsorbed on silver—Herzberg-Teller contribution. J. Phys. Chem. 1994, 98, 12702–12707. [Google Scholar] [CrossRef]
- Suzuki, S.; Kaneko, S.; Fujii, S.; Marqués-González, S.; Nishino, T.; Kiguchi, M. Effect of the molecule-metal interface on the surface-enhanced Raman scattering of 1,4-benzenedithiol. J. Phys. Chem. C 2016, 120, 1038–1042. [Google Scholar] [CrossRef]
- Okoli, C.P.; Naidoo, E.B.; Ofomaja, A.E. Role of synthesis process variables on magnetic functionality, thermal stability, and tetracycline adsorption by magnetic starch nanocomposite. Environ. Nanotechnol. Monit. Manag. 2018, 9, 141–153. [Google Scholar] [CrossRef]
- Mecheri, B.; Gambinossi, F.; Nocentini, M.; Puggelli, M.; Caminati, G. Modulation of tetracycline-phospholipid interactions by tuning of pH at the water-air interface. Biophys. Chem. 2004, 111, 15–26. [Google Scholar] [CrossRef]
- Morandi, S.; Focardi, C.; Nocentini, M.; Puggelli, M.; Caminati, G. Development and validation of europium-sensitized luminescence (ESL) method for the determination of tetracycline residues in milk. Food Anal. Methods 2009, 2, 271–281. [Google Scholar] [CrossRef]











| Raman (cm−1) | SERS (cm−1) | Assignment |
|---|---|---|
| 1619 | ----- | υ(CO1) + υ(amid-CO) + δ(amid-NH) + υ(CO3) + υ(C2-C3) + υ(OH10,12) + υ(amid-CO2) |
| 1560 | ----- | (C11O) + (CC)D |
| 1447 | 1459 | δ(CH7,8,9) + δ(OH12) + υ(D) + υ(CO10,CO11,CO12) + δ(amin-CH3) + δ(CH36) |
| 1315 | 1347 | δ(OH10,12) + δ(CH4,4a,5,5a) + υ(C5aC11a) + υ(C1C2) + υ(C9C10,C10C10a,C10aC11) + υ(CO11,12) + υ(CO3) + δ(CH7,8,9) |
| 1286 | 1283 | δ(CH4,4a,5a) + δ(OH12) + δ(amid-NH) + υ(CO10) + υ(CO3) + υ(CH7,8.9) + υ(amid-NC) + υ(C4aC5) + υ(D) |
| 1172 | 1175 | υ(CO3) |
| 1137 | 1133 | υ(CO6) + υ(CO12) |
| 859 | 888 | δ(CC)A,B,C,D + υ(C15 O25 ) |
| 707 | 747 | ω(amid-NH), v(CO6,12), γ(OH3,6,10,12,12a) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, P.C.; Fateixa, S.; Nogueira, H.I.S.; Trindade, T. Magnetite-Supported Gold Nanostars for the Uptake and SERS Detection of Tetracycline. Nanomaterials 2019, 9, 31. https://doi.org/10.3390/nano9010031
Pinheiro PC, Fateixa S, Nogueira HIS, Trindade T. Magnetite-Supported Gold Nanostars for the Uptake and SERS Detection of Tetracycline. Nanomaterials. 2019; 9(1):31. https://doi.org/10.3390/nano9010031
Chicago/Turabian StylePinheiro, Paula C., Sara Fateixa, Helena I. S. Nogueira, and Tito Trindade. 2019. "Magnetite-Supported Gold Nanostars for the Uptake and SERS Detection of Tetracycline" Nanomaterials 9, no. 1: 31. https://doi.org/10.3390/nano9010031
APA StylePinheiro, P. C., Fateixa, S., Nogueira, H. I. S., & Trindade, T. (2019). Magnetite-Supported Gold Nanostars for the Uptake and SERS Detection of Tetracycline. Nanomaterials, 9(1), 31. https://doi.org/10.3390/nano9010031