Three-Dimensional Stable Alginate-Nanocellulose Gels for Biomedical Applications: Towards Tunable Mechanical Properties and Cell Growing
Abstract
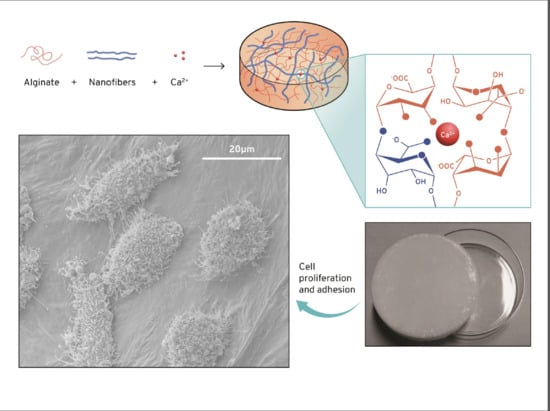
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Extraction and Functionalization of the Nanocelluloses
Preparation of Cellulose Nanofibers
Preparation of Cellulose Nanocrystals (CNC)
Preparation of TEMPO-Oxidized Cellulose Nanofibers (CNFTs)
Preparation of TEMPO-Oxidized Cellulose Nanocrystals (CNCTs)
2.2.2. Preparation of the Alginate and Nanocellulose Gels
2.2.3. Determination of the Equilibrium Time and Moisture Uptake of the Gels
2.2.4. Cell Growth and Cytotoxicity Tests of Alginate and Nanocelluloses Gels
Cell Culture
In Vitro Cytotoxicity Test
Cell Proliferation and Bioadhesion
2.2.5. Characterization Methods
3. Results and Discussion
3.1. Morphological, Structural, and Chemical Properties of Nanocelluloses
3.2. Pure Alginate and Nanocellulose-Alginate Gels
3.3. Cytotoxicity and Cell Growth Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hennink, W.E.; Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 13–36. [Google Scholar] [CrossRef]
- Joddar, B.; Garcia, E.; Casas, A.; Stewart, C.M. Development of functionalized multi-walled carbon-nanotube based alginate hydrogels for enabling biomimetic technologies. Sci. Rep. 2016, 6, 32456. [Google Scholar] [CrossRef]
- Frampton, J.P.; Hynd, M.R.; Shuler, M.L.; Shain, W. Fabrication and optimization of alginate hydrogel constructs for use in 3D neural cell culture. Biomed. Mater. 2011, 6, 015002. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Matricardi, P.; Di Meo, C.; Coviello, T.; Hennink, W.E.; Alhaique, F. Interpenetrating polymer networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv. Drug Deliv. Rev. 2013, 65, 1172–1187. [Google Scholar] [CrossRef]
- Naseri, N.; Deepa, B.; Mathew, A.P.; Oksman, K.; Girandon, L. Nanocellulose-based interpenetrating polymer network (IPN) hydrogels for cartilage applications. Biomacromolecules 2016, 17, 3714–3723. [Google Scholar] [CrossRef]
- Ingber, D.E. Cellular mechanotransduction: Putting all the pieces together again. FASEB J. 2006, 20, 811–827. [Google Scholar] [CrossRef]
- Yang, J.; Han, C.R.; Duan, J.F.; Xu, F.; Sun, R.C. Mechanical and viscoelastic properties of cellulose nanocrystals reinforced poly(ethylene glycol) nanocomposite hydrogels. ACS Appl. Mater. Interfaces 2013, 5, 3199–3207. [Google Scholar] [CrossRef]
- Elbert, D.L. Bottom-up tissue engineering. Curr. Opin. Biotechnol. 2011, 22, 674–680. [Google Scholar] [CrossRef] [Green Version]
- Jungst, T.; Smolan, W.; Schacht, K.; Scheibel, T.; Groll, J. Strategies and molecular design criteria for 3D printable hydrogels. Chem. Rev. 2016, 116, 1496–1539. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Huang, L.; Wang, Z.; Wang, L. Design and performance of a sericin-alginate interpenetrating network hydrogel for cell and drug delivery. Sci. Rep. 2015, 5, 12374. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, R.S.; Moura, M.R.; Aouada, F.A. Otimização da síntese de hidrogéis nanocompósitos intercalados para possível aplicação na área médica. Quim. Nova 2017, 40, 60–67. [Google Scholar] [CrossRef]
- Rashad, A.; Mustafa, K.; Heggset, E.B.; Syverud, K. Cytocompatibility of wood-derived cellulose nanofibril hydrogels with different surface chemistry. Biomacromolecules 2017, 18, 1238–1248. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G. Potential and limitations of nanocelluloses as components in biocomposite inks for three-dimensional bioprinting and for biomedical devices. Biomacromolecules 2018, 19, 701–711. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Development of sodium alginate-xanthan gum based nanocomposite scaffolds reinforced with cellulose nanocrystals and halloysite nanotubes. Polym. Test. 2017, 63, 214–225. [Google Scholar] [CrossRef]
- Park, M.; Lee, D.; Hyun, J. Nanocellulose-alginate hydrogel for cell encapsulation. Carbohydr. Polym. 2015, 116, 223–228. [Google Scholar] [CrossRef]
- Nordli, H.R.; Chinga-Carrasco, G.; Rokstad, A.M.; Pukstad, B. Producing ultrapure wood cellulose nanofibrils and evaluating thecytotoxicity using human skin cells. Carbohydr. Polym. 2016, 150, 65–73. [Google Scholar] [CrossRef]
- Basu, A.; Lindh, J.; Ålander, E.; Strømme, M.; Ferraz, M. On the use of ion-crosslinked nanocellulose hydrogels for wound healing solutions: Physicochemical properties and application-oriented biocompatibility studies. Carbohydr. Polym. 2017, 174, 299–308. [Google Scholar] [CrossRef]
- Hakkarainen, T.; Koivuniemi, R.; Kosonen, M.; Escobedo-Lucea, C.; Sanz-Garcia, A.; Vuola, J.; Valtonen, J.; Tammela, P.; Mäkitie, A.; Luukko, K.; et al. Nanofibrillar cellulose wound dressing in skin graft donor site treatment. J. Control. Release 2016, 244, 292–301. [Google Scholar] [CrossRef] [Green Version]
- Malinen, M.M.; Kanninen, L.K.; Corlu, A.; Isoniemi, H.M.; Lou, Y.R.; Yliperttula, M.L.; Urtti, A.O. Differentiation of liver progenitor cell line to functional organotypic cultures in 3D nanofibrillar cellulose and hyaluronan-gelatin hydrogels. Biomaterials 2014, 35, 5110–5121. [Google Scholar] [CrossRef] [Green Version]
- Lin, N.; Bruzzese, C.; Dufresne, A. TEMPO-oxidized nanocellulose participating as crosslinking aid for alginate-based sponges. ACS Appl. Mater. Interfaces 2012, 4, 4948–4959. [Google Scholar] [CrossRef]
- Aarstad, O.; Heggset, E.B.; Pedersen, I.S.; Bjørnøy, H.S.; Syverud, K.; Strand, B.L. Mechanical properties of composite hydrogels of alginate and cellulose nanofibrils. Polymers 2017, 9, 378. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of hydrogels and aerogels containing nanocellulose. Chem. Rev. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Wang, K.; Nune, K.; Misra, R. The functional response of alginate-gelatin-nanocrystalline cellulose injectable hydrogels toward delivery of cells and bioactive molecules. Acta Biomater. 2016, 36, 143–151. [Google Scholar] [CrossRef]
- Alexandrescu, L.; Syverud, K.; Gatti, A.; Chinga-Carrasco, G. Cytotoxicity tests of cellulose nanofibril-based structures. Cellulose 2013, 20, 1765–1775. [Google Scholar] [CrossRef]
- Colic, M.; Mihajlovic, D.; Mathew, A.; Naseri, N.; Kokol, V. Cytocompatibility and immunomodulatory properties of wood based nanofibrillated cellulose. Cellulose 2015, 22, 763–778. [Google Scholar] [CrossRef]
- Catalán, J.; Ilves, M.; Jarventaus, J.; Hannukainen, K.-S.; Kontturi, E.; Vanhala, E.; Alenius, H.; Savolainen, K.M.; Norppa, H. Genotoxic and immunotoxic effects of cellulose nanocrystals in vitro. Environ. Mol. Mutagen. 2015, 56, 171–182. [Google Scholar] [CrossRef]
- Lopes, V.R.; Sanchez-Martinez, C.; Strømme, M.; Ferraz, N. In vitro biological responses to nanofibrillated cellulose by humandermal, lungand immunecells: Surface chemistry aspect. Part. Fibre Toxicol. 2017, 14, 1. [Google Scholar] [CrossRef]
- Liu, J.; Bacher, M.; Rosenau, T.; Willför, S.; Mihranyan, A. Potentially immunogenic contaminants in wood-based and bacterial nanocellulose: Assessment of endotoxin and (1,3) β-d-Glucan Leves. Biomacromolecules 2018, 19, 150–157. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline celulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native celulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Isogai, A. TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- Pariente, J.L.; Kim, B.S.; Atala, A. In vitro biocompatibility evaluation of naturally derived and synthetic biomaterials using normal human bladder smooth muscle cells. J. Urol. 2002, 167, 1867–1871. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Gomes, M.E.; Reis, R.L. The potential of cellulose nanocrystals in tissue engineering strategies. Biomacromolecules 2014, 15, 2327–2346. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.; Creely, J.; Martin, A.; Conrad, C. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Chang, P.R.; Feng, J.; Yu, J. Surface acetylation of celulose nanocrystal and its reinforcing function in poly(lactic acid). Carbohydr. Polym. 2011, 83, 1834–1842. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A new ageless Bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Sehaqui, H.; Mushi, N.E.; Morimune, S.; Salajkova, M.; Nishino, T.; Berglund, L.A. Cellulose nanofiber orientation in nanopaper and nanocomposites by cold drawing. ACS Appl. Mater. Interfaces 2012, 4, 1043–1049. [Google Scholar] [CrossRef]
- Reddy, K.O.; Guduri, B.R.; Rajulu, A.V. Structural characterization and tensile properties of borassus fruit fibers. J. Appl. Polym. Sci. 2009, 114, 603–611. [Google Scholar] [CrossRef]
- Podsiadlo, P.; Choi, S.Y.; Shim, B.; Lee, J.; Cuddihy, M.; Kotov, N.A. Molecularly engineered nanocomposites: Layer-by-layer assembly of cellulose nanocrystals. Biomacromolecules 2005, 6, 2914–2918. [Google Scholar] [CrossRef]
- Lahiji, R.R.; Xu, X.; Reifenberger, R.; Raman, A.; Rudie, A.; Moon, R.J. Atomic force microscopy characterization of cellulose nanocrystals. Langmuir 2010, 26, 4480–4488. [Google Scholar] [CrossRef] [PubMed]
- Pääkö, M.; Ankerfors, M.; Kosonen, H.; Nykanen, A.; Ahola, S.; Osterberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; Lindstrom, T. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.L. Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohydr. Polym. 2013, 95, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Chanzy, H.; Vignon, M. TEMPO-mediated surface oxidation of cellulose whiskers. Cellulose 2006, 13, 679–687. [Google Scholar] [CrossRef] [Green Version]
- Taqieddin, E.; Amiji, M. Enzyme immobilization in novel alginate-chitosan core-shell microcapsules. Biomaterials 2004, 25, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Balakrishinan, B.; Mohanty, M.; Umashar, P.R.; Jayakrishnan, A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 2005, 26, 6335–6342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, J.; Chang, P.R.; Li, J.; Chen, Y.; Wang, D.; Yu, J.; Chen, J. Structure and properties of polysaccharide nanocrystal-doped supramolecular hydrogels based on cyclodextrin inclusion. Polymer 2010, 51, 4398–4407. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Q.; Yue, Y.; Zhang, Q.J. Application of rod-shaped cellulose nanocrystals in polyacrylamide hydrogels. Colloid Interface Sci. 2011, 353, 116–123. [Google Scholar] [CrossRef]
- Smitha, B.; Sridhar, S.; Khan, A.A. Chitosan-sodium alginate polyion complexes as fuel cell membranes. Eur. Polym. J. 2005, 41, 1859–1866. [Google Scholar] [CrossRef]
- Sartori, C.; Finch, D.S.; Ralph, B.E.; Gilding, K. Determination of the cation content of alginate thin films by FTIR spectroscopy. Polymer 1997, 38, 43–51. [Google Scholar] [CrossRef]
- Sehaqui, H.; Zhou, Q.; Berglund, L.A. High-porosity aerogels of high specific surface area prepared from nanofibrillated cellulose (NFC). Compos. Sci. Technol. 2011, 71, 1593–1599. [Google Scholar] [CrossRef]
- Wake, M.C.; Patrick, C.W., Jr.; Mikos, A.G. Pore morphology effects on the fibrovascular tissue growth in porous polymer substrates. Cell Transplant. 1994, 3, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ramay, H.R.; Hauch, K.D.; Xiao, D.; Zhang, M. Chitosan-alginate hybrid scaffolds for bone tissue engineering. Biomaterials 2005, 26, 3919–3928. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.K.; Anjali, G. Water sorption behaviour of highly swelling (carboxy-methylcellulose-g-polyacrylamide) hydrogels and release of potassium nitrate as agrochemical. Carbohydr. Polym. 2003, 53, 271–279. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Breger, J.C.; Fisher, B.; Samy, R.; Pollack, S.; Wang, N.S.; Isayeva, I.J. Synthesis of “click” alginate hydrogel capsules and comparison of their stability, water swelling, and diffusion properties with that of Ca2+ crosslinked alginate capsules. Biomed. Mater. Res. Part B 2015, 103, 1120–1132. [Google Scholar] [CrossRef]
- Zander, N.E.; Dong, H.; Steele, J.; Grant, J.T. Metal cation crosslinked nanocellulose hydrogels as tissue engineering substrates. ACS Appl. Mater. Interfaces 2014, 6, 18502–18510. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Abreu, F.O.M.; Bianchini, C.; Forte, A.A.C.; Kist, T.B.L. Influence of the composition and preparation method on the morphology and swelling behavior of alginate-chitosan hydrogels. Carbohydr. Polym. 2008, 74, 283–289. [Google Scholar] [CrossRef]
- Li, X.; Xie, H.; Lin, J.; Xie, W.; Ma, X. Characterization and biodegradation of chitosan-alginate polyelectrolyte complexes. Polym. Degrad. Stab. 2009, 94, 1–6. [Google Scholar] [CrossRef]
- Han, J.; Zhou, Z.; Yin, R.; Yang, D.; Nie, J. Alginate-chitosan/hydroxiapatite polyelectrolyte complex porous scaffolds: Preparation and characterization. Int. J. Biol. Macromol. 2009, 46, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, B.; Turi, E.A. Thermal Characterization of Polymer Materials, 2nd ed.; Academic Press Inc.: New York, NY, USA, 1997; Volume 1, p. 305. [Google Scholar]
- Hutchinson, J.; Haward, R.N.; Young, R.J. The Physics of Glassy Polymers, 2nd ed.; Chapman and Hall: London, UK, 1997; p. 85. [Google Scholar]
- Kirdponpattara, S.; Khamkeaw, A.; Sanchavanakit, N.; Pavasant, P.; Phisalaphong, M. Structural modification and characterization of bacterial cellulose-alginate composite scaffolds for tissue engineering. Carbohydr. Polym. 2015, 132, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.X.; Dai, L.B.; Shi, H.Z.; Xiong, S.; Zhou, C.R. In vitro evaluation of alginate/halloysite nanotube composite scaffolds for tissue engineering. Mater. Sci. Eng. C 2015, 49, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Skaugrud, O.; Hagen, A.; Borgersen, B.; Dornish, M. Biomedical and pharmaceutical applications of alginate and chitosan. Biotechnol. Genet. Eng. Rev. 1999, 16, 23–40. [Google Scholar] [CrossRef] [PubMed]







| Sample Name | Alginate (g) | Nanocellulose (g) | Nanocellulose/Alginate Weight Ratio |
|---|---|---|---|
| AlgCa | 0.200 | 0.000 | 0.000 |
| AlgCNC10 | 0.281 | 0.030 | 0.100 |
| AlgCNC36 | 0.200 | 0.112 | 0.560 |
| AlgCNC50 | 0.156 | 0.156 | 1.000 |
| AlgCNCT10 | 0.281 | 0.030 | 0.100 |
| AlgCNCT36 | 0.200 | 0.112 | 0.560 |
| AlgCNCT50 | 0.156 | 0.156 | 1.000 |
| AlgCNF10 | 0.281 | 0.030 | 0.100 |
| AlgCNF36 | 0.200 | 0.112 | 0.560 |
| AlgCNF50 | 0.156 | 0.156 | 1.000 |
| AlgCNFT10 | 0.281 | 0.030 | 0.100 |
| AlgCNFT36 | 0.200 | 0.112 | 0.560 |
| AlgCNFT50 | 0.156 | 0.156 | 1.000 |
| Sample | Moisture Uptake (g/g) Milli-Q H2O; pH 6.2 | Moisture Uptake (g/g) PBS; pH 7.4 |
|---|---|---|
| AlgCa | 6.64 ± 0.05 | 10.75 ± 0.62 |
| AlgCNC10 | 5.86 ± 0.20 | 9.16 ± 0.91 |
| AlgCNC36 | 8.33 ± 0.05 | 8.52 ± 0.59 |
| AlgCNC50 | 7.47 ± 0.46 | 8.89 ± 1.95 |
| AlgCNCT10 | 7.57 ± 0.77 | 7.35 ± 0.68 |
| AlgCNCT36 | 8.44 ± 0.45 | 9.01 ± 1.28 |
| AlgCNCT50 | 8.24 ± 0.51 | 11.45 ± 0.50 |
| AlgCNF10 | 6.86 ± 0.13 | 11.45 ± 0.42 |
| AlgCNF36 | 9.78 ± 0.10 | 11.50 ± 0.56 |
| AlgCNF50 | 11.36 ± 0.61 | 13.19 ± 2.00 |
| AlgCNFT10 | 16. 31 ± 0.58 | 17.34 ± 0.85 |
| AlgCNFT36 | 17.73 ± 0.78 | 17.72 ± 0.86 |
| AlgCNFT50 | 14.61 ± 0.06 | 18.13 ± 0.56 |
| SAMPLE | 35 °C | 37 °C | 42 °C | |||
|---|---|---|---|---|---|---|
| E’1 (MPa) | E’2 (MPa) | E’1 (MPa) | E’2 (MPa) | E’1 (MPa) | E’2 (MPa) | |
| AlgCNC36 | 2.27 | 2.12 | 2.67 | 2.63 | 3.00 | 3.02 |
| AlgCNC50 | 0.10 | 0.12 | 0.10 | 0.11 | 0.10 | 0.12 |
| AlgCNCT36 | 4.49 | 4.48 | 5.25 | 5.24 | 7.83 | 7.81 |
| AlgCNCT50 | 0.97 | 0.96 | 1.14 | 1.12 | 1.22 | 1.21 |
| AlgCNF36 | 0.15 | 2.13 | 0.16 | 2.10 | 0.47 | 2.29 |
| AlgCNF50 | 0.02 | 0.23 | 0.02 | 0.24 | 0.02 | 0.22 |
| AlgCNFT36 | 1.13 | 2.52 | 1.30 | 2.55 | 1.66 | 2.66 |
| AlgCNFT50 | 0.33 | 3.46 | 0.40 | 3.54 | 0.62 | 3.74 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siqueira, P.; Siqueira, É.; De Lima, A.E.; Siqueira, G.; Pinzón-Garcia, A.D.; Lopes, A.P.; Segura, M.E.C.; Isaac, A.; Pereira, F.V.; Botaro, V.R. Three-Dimensional Stable Alginate-Nanocellulose Gels for Biomedical Applications: Towards Tunable Mechanical Properties and Cell Growing. Nanomaterials 2019, 9, 78. https://doi.org/10.3390/nano9010078
Siqueira P, Siqueira É, De Lima AE, Siqueira G, Pinzón-Garcia AD, Lopes AP, Segura MEC, Isaac A, Pereira FV, Botaro VR. Three-Dimensional Stable Alginate-Nanocellulose Gels for Biomedical Applications: Towards Tunable Mechanical Properties and Cell Growing. Nanomaterials. 2019; 9(1):78. https://doi.org/10.3390/nano9010078
Chicago/Turabian StyleSiqueira, Priscila, Éder Siqueira, Ana Elza De Lima, Gilberto Siqueira, Ana Delia Pinzón-Garcia, Ana Paula Lopes, Maria Esperanza Cortés Segura, Augusta Isaac, Fabiano Vargas Pereira, and Vagner Roberto Botaro. 2019. "Three-Dimensional Stable Alginate-Nanocellulose Gels for Biomedical Applications: Towards Tunable Mechanical Properties and Cell Growing" Nanomaterials 9, no. 1: 78. https://doi.org/10.3390/nano9010078
APA StyleSiqueira, P., Siqueira, É., De Lima, A. E., Siqueira, G., Pinzón-Garcia, A. D., Lopes, A. P., Segura, M. E. C., Isaac, A., Pereira, F. V., & Botaro, V. R. (2019). Three-Dimensional Stable Alginate-Nanocellulose Gels for Biomedical Applications: Towards Tunable Mechanical Properties and Cell Growing. Nanomaterials, 9(1), 78. https://doi.org/10.3390/nano9010078