Interactions of Nanoparticles and Biosystems: Microenvironment of Nanoparticles and Biomolecules in Nanomedicine
Abstract
:1. Introduction
2. Nanoparticle-Cell Dynamics
2.1. Cellular Internalization
2.2. Tumor Accumulation
2.3. Elimination
3. Nanoparticle Interactions
3.1. Interaction Mechanisms Between Nanoparticles and Biomolecules
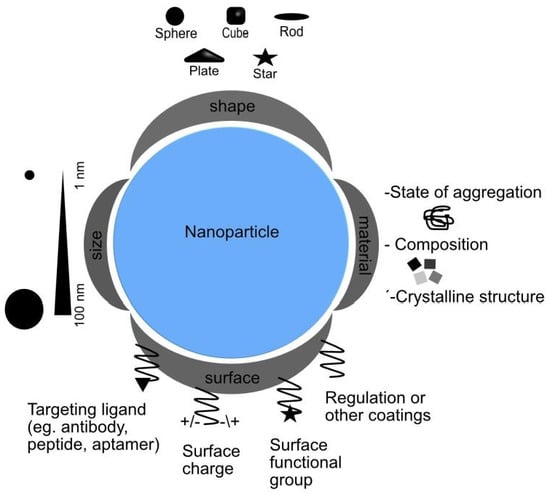
3.2. Nanoparticle Design: Influence on Interaction Mechanisms
3.2.1. Size
3.2.2. Shape
3.2.3. Surface Modification
3.2.4. Chemical Composition
3.2.5. Protein Corona
4. Applications of Nanoparticles
Nanomedical Applications: Immunotherapy
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dean, S.; Mansoori, G.; Fauzi Soelaiman, T. Nanotechnology—An Introduction for the Standards Community. J. ASTM Int. 2005, 2, 13110. [Google Scholar] [CrossRef]
- Schmidt, K.F. Nanofrontiers: Visions for the Future of Nanotechnology; Woodrow Wilson International Center for Scholars: Washington, DC, USA, 2007. [Google Scholar]
- National Nanotechnology Initiative. Available online: http://www.nano.gov (accessed on 14 June 2019).
- Davies, J.C. Nanotechnology Oversight; Project on Emerging Nanotechnologies: Washington, DC, USA, 2008. [Google Scholar]
- Bera, A.; Belhaj, H. Application of Nanotechnology by Means of Nanoparticles and Nanodispersions in Oil Recovery—A Comprehensive Review. J. Nat. Gas Sci. Eng. 2016, 34, 1284–1309. [Google Scholar] [CrossRef]
- Riehemann, K.; Schneider, S.W.; Luger, T.A.; Godin, B.; Ferrari, M.; Fuchs, H. Nanomedicine-Challenge and Perspectives. Angew. Chem. Int. Ed. 2009, 48, 872–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, F.; Sobolev, K. Nanotechnology in Concrete-A Review. Constr. Build. Mater. 2010, 24, 2060–2071. [Google Scholar] [CrossRef]
- Ingle, A.P.; Duran, N.; Rai, M. Bioactivity, Mechanism of Action, and Cytotoxicity of Copper-Based Nanoparticles: A Review. Appl. Microbiol. Biotechnol. 2014, 98, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Stellacci, F. Effect of Surface Properties on Nanoparticle-Cell Interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Jyoti, K.; Patnaik, A.; Singh, A.; Chauhan, R.; Chandel, S.S. Biosynthesis, Characterization and Antibacterial Activity of Silver Nanoparticles Using an Endophytic Fungal Supernatant of Raphanus Sativus. J. Genet. Eng. Biotechnol. 2017, 15, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Phogat, N.; Kohl, M.; Uddin, I.; Jahan, A. Interaction of Nanoparticles With Biomolecules, Protein, Enzymes, and Its Applications. In Precision Medicine; Elsevier: Atlanta, GA, USA, 2018; pp. 253–276. [Google Scholar]
- Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface Modifications of Nanoparticles for Stability in Biological Fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef]
- Dutta, P.P.; Bordoloi, M.; Gogoi, K.; Roy, S.; Narzary, B.; Bhattacharyya, D.R.; Mohapatra, P.K.; Mazumder, B. Antimalarial Silver and Gold Nanoparticles: Green Synthesis, Characterization and in Vitro Study. Biomed. Pharmacother. 2017, 91, 567–580. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Jain, P. Biomolecules–Nanoparticles: Interaction in Nanoscale. In Metal Nanoparticles in Microbiology; Rai, M., Duran, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 135–150. [Google Scholar]
- Byrne, H.J.; Ahluwalia, A.; Boraschi, D.; Fadeel, B.; Gehr, P.; Gutleb, A.C.; Kendall, M.; Papadopoulos, M.G. The bio-nano-interface in predicting nanoparticle fate and behaviour in living organisms: Towards grouping and categorising nanomaterials and ensuring nanosafety by design. BioNanoMaterials 2013, 14, 195–216. [Google Scholar]
- Yameen, B.; Choi, W.I.; Vilos, C.; Swami, A.; Shi, J.; Farokhzad, O.C. Insight into Nanoparticle Cellular Uptake and Intracellular Targeting. J. Control. Release 2014, 190, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Jiao, B.; Shi, X.; Valle, R.P.; Fan, Q.; Zuo, Y.Y. Physicochemical Properties of Nanoparticles Regulate Translocation across Pulmonary Surfactant Monolayer and Formation of Lipoprotein Corona. ACS Nano 2013, 7, 10525–10533. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Nienhaus, K.; Nienhaus, G. Engineered Nanoparticles Interacting with Cells: Size Matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Stenzel, M.H. Entry of Nanoparticles into Cells: The Importance of Nanoparticle Properties. Polym. Chem. 2018, 9, 259–272. [Google Scholar] [CrossRef]
- Wilhelm, C.; Billotey, C.; Roger, J.; Pons, J.N.; Bacri, J.; Gazeau, F. Intracellular Uptake of Anionic Superparamagnetic Nanoparticles as a Function of Their Surface Coating. Biomaterials 2003, 24, 1001–1011. [Google Scholar] [CrossRef]
- Champion, J.A.; Mitragotri, S. Role of Target Geometry in Phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Toward a Full Understanding of the EPR Effect in Primary and Metastatic Tumors as Well as Issues Related to Its Heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and Nanoparticles: Sources and Toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef]
- Pirollo, K.F.; Chang, E.H. Does a Targeting Ligand Influence Nanoparticle Tumor Localization or Uptake? Trends Biotechnol. 2008, 26, 552–558. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor Vascular Permeability and the EPR Effect in Macromolecular Therapeutics: A Review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Ernsting, M.J.; Murakami, M.; Roy, A.; Li, S.-D. Factors Controlling the Pharmacokinetics, Biodistribution and Intratumoral Penetration of Nanoparticles. J. Control. Release 2013, 172, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H.; Nagasaki, Y.; Kataoka, K. PEGylated Nanoparticles for Biological and Pharmaceutical Applications. Adv. Drug Deliv. Rev. 2003, 55, 403–419. [Google Scholar] [CrossRef]
- Boczkowski, J.; Lanone, S. Respiratory Toxicities of Nanomaterials—A Focus on Carbon Nanotubes. Adv. Drug Deliv. Rev. 2012, 64, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Jiang, G.; Chen, L.; Zhou, H.; Fourches, D.; Tropsha, A.; Yan, B. Chemical Basis of Interactions Between Engineered Nanoparticles and Biological Systems. Chem. Rev. 2014, 114, 7740–7781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, C.P.; Abdul-Wahab, M.F.; Jaafar, J.; Chan, G.F.; Rashid, N.A.A. Effect of PH and Biological Media on Polyvinylpyrrolidone-Capped Silver Nanoparticles. AIP Conf. Proc. 2016, 1756, 1–8. [Google Scholar]
- Carrillo-Carrión, C.; Nazarenus, M.; Paradinas, S.S.; Carregal-Romero, S.; Almendral, M.J.; Fuentes, M.; Pelaz, B.; del Pino, P.; Hussain, I.; Clift, M.J.; et al. Metal Ions in the Context of Nanoparticles toward Biological Applications. Curr. Opin. Chem. Eng. 2014, 4, 88–96. [Google Scholar] [CrossRef]
- Sharma, V.K.; Sayes, C.M.; Guo, B.; Pillai, S.; Parsons, J.G.; Wang, C.; Yan, B.; Ma, X. Interactions between Silver Nanoparticles and Other Metal Nanoparticles under Environmentally Relevant Conditions: A Review. Sci. Total Environ. 2019, 653, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Peer, D. Immunotoxicity Derived from Manipulating Leukocytes with Lipid-Based Nanoparticles. Adv. Drug Deliv. Rev. 2012, 64, 1738–1748. [Google Scholar] [CrossRef]
- Albanese†, A.; Chang, W.C. Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano 2011, 57, 5478–5489. [Google Scholar] [CrossRef]
- Nichols, G.; Byard, S.; Bloxham, M.J.; Botterill, J.; Dawson, N.J.; Dennis, A.; Diart, V.; North, N.C.; Sherwood, J.D. A Review of the Terms Agglomerate and Aggregate with a Recommendation for Nomenclature Used in Powder and Particle Characterization. J. Pharm. Sci. 2002, 91, 2103–2109. [Google Scholar] [CrossRef]
- Pellegrino, F.; Pellutiè, L.; Sordello, F.; Minero, C.; Ortel, E.; Hodoroaba, V.D.; Maurino, V. Influence of Agglomeration and Aggregation on the Photocatalytic Activity of TiO2 Nanoparticles. Appl. Catal. B Environ. 2017, 216, 80–87. [Google Scholar] [CrossRef]
- Zook, J.M.; MacCuspie, R.I.; Locascio, L.E.; Halter, M.D.; Elliott, J.T. Stable Nanoparticle Aggregates/Agglomerates of Different Sizes and the Effect of Their Size on Hemolytic Cytotoxicity. Nanotoxicology 2011, 5, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Wang, T. A New View for Nanoparticle Assemblies: From Crystalline to Binary Cooperative Complementarity. Chem. Soc. Rev. 2017, 46, 1483–1509. [Google Scholar] [CrossRef] [PubMed]
- Singamaneni, S.; Bliznyuk, V.N.; Binek, C.; Tsymbal, E.Y. Magnetic Nanoparticles: Recent Advances in Synthesis, Self-Assembly and Applications. J. Mater. Chem. 2011, 21, 16819. [Google Scholar] [CrossRef]
- Mørup, S.; Hansen, M.F.; Frandsen, C. Magnetic Interactions between Nanoparticles. Beilstein J. Nanotechnol. 2010, 1, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Dyawanapelly, S.; Mehrotra, P.; Ghosh, G.; Jagtap, D.D.; Dandekar, P.; Jain, R. How the Surface Functionalized Nanoparticles Affect Conformation and Activity of Proteins: Exploring through Protein-Nanoparticle Interactions. Bioorg. Chem. 2019, 82, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Gehr, P. Interaction of Nanoparticles with Biological Systems. Colloids Surf. B Biointerfaces 2018, 172, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Firkowska-Boden, I.; Zhang, X.; Jandt, K.D. Controlling Protein Adsorption through Nanostructured Polymeric Surfaces. Adv. Healthc. Mater. 2018, 7, 1700995. [Google Scholar] [CrossRef] [PubMed]
- Hemmelmann, P. Nanoparticles, Proteins, and Nucleic Acids: Biotechnology Meets Materials Science. Angew. Chem. Int. Ed. 2001, 40, 4128–4158. [Google Scholar]
- Arsalan, A.; Younus, H. Enzymes and Nanoparticles: Modulation of Enzymatic Activity via Nanoparticles. Int. J. Biol. Macromol. 2018, 118, 1833–1847. [Google Scholar] [CrossRef]
- Saallah, S.; Lenggoro, I.W. Nanoparticles Carrying Biological Molecules: Recent Advances and Applications. KONA Powder Part. J. 2018, 2018, 89–111. [Google Scholar] [CrossRef]
- Singh, B.; Mitragotri, S. Harnessing Cells to Deliver Nanoparticle Drugs to Treat Cancer. Biotechnol. Adv. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ukidve, A.; Krishnan, V.; Mitragotri, S. Effect of Physicochemical and Surface Properties on in Vivo Fate of Drug Nanocarriers. Adv. Drug Deliv. Rev. 2019, 143, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Centi, S.; Tatini, F.; Ratto, F.; Gnerucci, A.; Mercatelli, R.; Romano, G.; Landini, I.; Nobili, S.; Ravalli, A.; Marrazza, G.; et al. In Vitro Assessment of Antibody-Conjugated Gold Nanorods for Systemic Injections. J. Nanobiotechnol. 2014, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Tatini, F.; National, I.; Massai, L. Size Dependent Biological Profiles of PEGylated Gold Nanorods. J. Mater. Chem. B 2014, 36, 6072–6080. [Google Scholar] [CrossRef]
- Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Mulvaney, P. Gold Nanorods: Synthesis, Characterization and Applications. Coord. Chem. Rev. 2005, 249, 1870–1901. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2017. [Google Scholar] [CrossRef]
- Aillon, K.L.; Xie, Y.; El-Gendy, N.; Berkland, C.J.; Forrest, M.L. Effects of Nanomaterial Physicochemical Properties on in Vivo Toxicity. Adv. Drug Deliv. Rev. 2009, 61, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y. The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms. Int. J. Mol. Sci. 2019, 18, 2702. [Google Scholar] [CrossRef] [PubMed]
- Id, M.A.; Moosavi, M.A.; Rahmati, M.; Falahati, M. Health Concerns of Various Nanoparticles: A Review of Their in Vitro and in Vivo Toxicity. Nanomaterials 2018, 8, 634. [Google Scholar] [Green Version]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The Effect of Nanoparticle Size on in Vivo Pharmacokinetics and Cellular Interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Albanese, A.; Tang, P.S.; Chan, W.C.W. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu. Rev. Biomed. Eng. 2014, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ballou, B.; Lagerholm, B.C.; Ernst, L.A.; Bruchez, M.P.; Waggoner, A.S. Noninvasive Imaging of Quantum Dots in Mice. Bioconjug. Chem. 2004, 15, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Sonavane, G.; Tomoda, K.; Makino, K. Biodistribution of Colloidal Gold Nanoparticles after Intravenous Administration: Effect of Particle Size. Colloids Surf. B Biointerfaces 2008, 66, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Ahn, E.; Park, Y. Shape-Dependent Cytotoxicity and Cellular Uptake of Gold Nanoparticles Synthesized Using Green Tea Extract. Nanoscale Res. Lett. 2019, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Risom, L.; Møller, P.; Loft, S. Oxidative Stress-Induced DNA Damage by Particulate Air Pollution. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 592, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Ospina, S.P.; Favi, P.M.; Gao, M.; Johana, L.; Morales, M.; Pavon, J.J.; Webster, T.J. Shape and Surface Effects on the Cytotoxicity of Nanoparticles: Gold Nanospheres versus Gold Nanostars. J. Biomed. Mater. Res. Part A 2015, 103, 3449–3462. [Google Scholar]
- Curtis, A.C.; Toghani, D.; Wong, B.; Nance, E. Colloidal stability as a determinant of nanoparticle behavior in the brain. Colloids Surf. B Biointerfaces 2018, 170, 673–682. [Google Scholar] [CrossRef]
- Hoshino, A.; Fujioka, K.; Oku, T.; Suga, M.; Sasaki, Y.F.; Ohta, T.; Yasuhara, M.; Suzuki, K.; Yamamoto, K. Physicochemical Properties and Cellular Toxicity of Nanocrystal Quantum Dots Depend on Their Surface Modification. Nano Lett. 2004, 4, 2163–2169. [Google Scholar] [CrossRef]
- Pietroiusti, A.; Massimiani, M.; Fenoglio, I.; Colonna, M.; Valentini, F.; Palleschi, G.; Camaioni, A.; Magrini, A.; Siracusa, G.; Bergamaschi, A.; et al. Low Doses of Pristine and Oxidized Single-Wall Carbon Nanotubes Affect Mammalian Embryonic Development. ACS Nano 2011, 5, 4624–4633. [Google Scholar] [CrossRef] [Green Version]
- Georgieva, J.V.; Kalicharan, D.; Couraud, P.; Romero, I.A.; Weksler, B.; Hoekstra, D.; Zuhorn, I.S. Surface Characteristics of Nanoparticles Determine Their Intracellular Fate in and Processing by Human Blood—Brain Barrier Endothelial Cells In Vitro. Mol. Ther. 2009, 19, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Kantner, X.K.; Geidel, X.C.; Brandholt, S.; De Cock, I.; Soenen, S.J.H.; Gil, P.R.; Montenegro, J.; Braeckmans, K.; Nienhaus, G.U.; et al. Polymer-Coated Nanoparticles Interacting with Proteins and Cells: Focusing on the Sign of the Net Charge. ACS Nano 2013, 7, 3253–3263. [Google Scholar]
- Chompoosor, A.; Saha, K.; Ghosh, P.S.; Macarthy, D.J.; Miranda, O.R.; Zhu, Z.-J.; Arcaro, K.F.; Rotello, V.M. The Role of Surface Functionality on Acute Cytotoxicity, ROS Generation and DNA Damage by Cationic Gold Nanoparticles. Small 2010, 6, 2246–2249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoudi, M.; Lynch, I.; Ejtehadi, M.R.; Monopoli, M.P.; Bombelli, F.B.; Laurent, S. Protein À Nanoparticle Interactions: Opportunities and Challenges. Chem. Rev. 2011, 111, 5610–5637. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, Y. Physicochemical Characteristics of Nanoparticles Affect Circulation, Biodistribution, Cellular Internalization, and Trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X.; Gu, N. Computational Investigation of Interaction between Nanoparticles and Membranes: Hydrophobic/Hydrophilic Effect. J. Phys. Chem. B 2008, 112, 16647–16653. [Google Scholar] [CrossRef] [PubMed]
- Griffitt, R.J.; Luo, J.; Gao, J.; Bonzongo, J.C.; Barber, D.S. Effects of Particle Composition and Species on Toxicity of Metallic Nanomaterials in Aquatic Organisms. Environ. Toxicol. Chem. 2008, 27, 1972–1978. [Google Scholar] [CrossRef]
- Gatoo, M.A.; Naseem, S.; Arfat, M.Y.; Mahmood Dar, A.; Qasim, K.; Zubair, S. Physicochemical Properties of Nanomaterials: Implication in Associated Toxic Manifestations. BioMed Res. Int. 2014, 2014, 498420. [Google Scholar] [CrossRef]
- Van Der Zande, M.; Vandebriel, R.J.; Van Doren, E.; Kramer, E.; Herrera Rivera, Z.; Serrano-Rojero, C.S.; Gremmer, E.R.; Mast, J.; Peters, R.J.B.; Hollman, P.C.H.; et al. Distribution, Elimination, and Toxicity of Silver Nanoparticles and Silver Ions in Rats after 28-Day Oral Exposure. ACS Nano 2012, 6, 7427–7442. [Google Scholar] [CrossRef]
- Peng, Q.; Zhang, S.; Yang, Q.; Zhang, T.; Wei, X.Q.; Jiang, L.; Zhang, C.L.; Chen, Q.M.; Zhang, Z.R.; Lin, Y.F. Preformed Albumin Corona, a Protective Coating for Nanoparticles Based Drug Delivery System. Biomaterials 2013, 34, 8521–8530. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Webster, T.J.; Kim, S. Effect of the Protein Corona on Nanoparticles for Modulating Cytotoxicity and Immunotoxicity. Int. J. Nanomed. 2015, 10, 97. [Google Scholar]
- Hirsh, S.L.; Mckenzie, D.R.; Nosworthy, N.J.; Denman, J.A.; Sezerman, O.U.; Bilek, M.M.M. Colloids and Surfaces B: Biointerfaces The Vroman Effect: Competitive Protein Exchange with Dynamic Multilayer Protein Aggregates. Colloids Surf. B Biointerfaces 2013, 103, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Megido, L.; Díez, P.; Fuentes, M. Nanoproteomics approaches in biomarcker Discovery: The critical role of protein corona on nanoparticles as drug carriers. In Nanotechnologies in Preventive and Regenerative Medicine; Uskokovic, V., Uskokovic, P.D., Eds.; Elsevier: Cambridge, UK, 2018; pp. 225–239. [Google Scholar]
- Liu, W.; Rose, J.; Plantevin, S.; Auffan, M.; Bottero, J.Y.; Vidaud, C. Protein Corona Formation for Nanomaterials and Proteins of a Similar Size: Hard or Soft Corona? Nanoscale 2013, 5, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Gao, H. The Impact of Protein Corona on the Behavior and Targeting Capability of Nanoparticle-Based Delivery System. Int. J. Pharm. 2018, 552, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Konduru, N.V.; Molina, R.M.; Swami, A.; Damiani, F.; Pyrgiotakis, G.; Lin, P.; Andreozzi, P.; Donaghey, T.C.; Demokritou, P.; Krol, S.; et al. Protein Corona: Implications for Nanoparticle Interactions with Pulmonary Cells. Part. Fibre Toxicol. 2017, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hadjidemetriou, M.; Mcadam, S.; Garner, G.; Thackeray, C.; Knight, D.; Smith, D.; Al-ahmady, Z.; Mazza, M.; Rogan, J. The Human In Vivo Biomolecule Corona onto PEGylated Liposomes: A Proof-of-Concept Clinical Study. Adv. Mater. 2018, 31, 1803335. [Google Scholar] [CrossRef]
- Del Pino, P.; Pelaz, B.; Zhang, Q.; Maffre, P.; Nienhaus, G.U.; Parak, W.J. Protein Corona Formation around Nanoparticles—From the Past to the Future. Mater. Horiz. 2014, 1, 301–313. [Google Scholar] [CrossRef]
- Strojan, K.; Leonardi, A.; Bregar, V.B.; Kriz, I. Dispersion of Nanoparticles in Different Media Importantly Determines the Composition of Their Protein Corona. PLoS ONE 2017, 12, e0169552. [Google Scholar] [CrossRef]
- Stark, W.J.; Stoessel, P.R.; Wohlleben, W.; Hafner, A. Industrial Applications of Nanoparticles. Chem. Soc. Rev. 2015, 44, 5793–5805. [Google Scholar] [CrossRef]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; Marei, H.E.S. Nanoparticles in Tissue Engineering: Applications, Challenges and Prospects. Int. J. Nanomed. 2018, 13, 5637–5655. [Google Scholar] [CrossRef]
- Danie Kingsley, J.; Ranjan, S.; Dasgupta, N.; Saha, P. Nanotechnology for Tissue Engineering: Need, Techniques and Applications. J. Pharm. Res. 2013, 7, 200–204. [Google Scholar] [CrossRef]
- Ellis-Behnke, R.G.; Liang, Y.X.; You, S.W.; Tay, D.K.; Zhang, S.; So, K.F.; Schneider, G.E. Nano neuro knitting: Peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision. Proc. Natl. Acad. Sci. USA 2006, 103, 5054. [Google Scholar] [CrossRef]
- De Kwaadsteniet, M.; Botes, M.; Cloete, T.E. Application of Nanotechnology in Antimicrobial Coatings in the Water Industry. Nano 2011, 6, 395–407. [Google Scholar] [CrossRef]
- Li, Y.; Leung, P.; Yao, L.; Song, Q.W.; Newton, E. Antimicrobial Effect of Surgical Masks Coated with Nanoparticles. J. Hosp. Infect. 2006, 62, 58–63. [Google Scholar] [CrossRef]
- Theivasanthi, T.; Alagar, M. Anti-Bacterial Studies of Silver Nanoparticles. arXiv 2011, arXiv:1101.0348. [Google Scholar]
- Kargozar, S.; Mozafari, M. Nanotechnology and Nanomedicine: Start Small, Think Big. Mater. Today Proc. 2018, 5, 15492–15500. [Google Scholar] [CrossRef]
- Carey, J.D. Engineering the next Generation of Large-Area Displays: Prospects and Pitfalls. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2003, 361, 2891–2907. [Google Scholar] [CrossRef]
- Roy, I.; Stachowiak, M.K.; Bergey, E.J. Nonviral Gene Transfection Nanoparticles: Function and Applications in the Brain. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 89–97. [Google Scholar] [CrossRef]
- Fatima, H.; Kim, K.S. Magnetic Nanoparticles for Bioseparation. Korean J. Chem. Eng. 2017, 34, 589–599. [Google Scholar] [CrossRef]
- Adeniyi, O.M.; Azimov, U.; Burluka, A. Algae biofuel: Current status and future applications. Renew. Sustain. Energy Rev. 2018, 90, 316–335. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Ouma, C.N.M.; du Preez, S.P.; Modisha, P.; Engelbrecht, N.; Bessarabov, D.G.; Ghimire, A. Application of Nanoparticles in Biofuels: An Overview. Fuel 2019, 237, 380–397. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J. Improving Mechanisms of Biohydrogen Production from Grass Using Zero-Valent Iron Nanoparticles. Bioresour. Technol. 2018, 266, 413–420. [Google Scholar] [CrossRef]
- Timothy, C.J.; Kogularamanan, S.; Sthepen, L. The Next Generation of Platinum Drugs: Targeted Pt (II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar]
- Díez, P.; González-Muñoz, M.; González-González, M.; Dégano, R.M.; Jara-Acevedo, R.; Sánchez-Paradinas, S.; Piñol, R.; Murillo, J.L.; Lou, G.; Palacio, F.; et al. Functional Insights into the Cellular Response Triggered by a Bile-Acid Platinum Compound Conjugated to Biocompatible Ferric Nanoparticles Using Quantitative Proteomic Approaches. Nanoscale 2017, 9, 9960–9972. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-Hydrogel: A Hybrid Biomaterial System for Localized Drug Delivery. Ann. Biomed. Eng. 2016, 44, 2049–2061. [Google Scholar] [CrossRef] [Green Version]
- Dizaj, S.M.; Barzegar-jalali, M.; Zarrintan, M.H.; Adibkia, K.; Lotfipour, F. Calcium Carbonate Nanoparticles as Cancer Drug Delivery System. Expert Opin. Drug Deliv. 2015, 12, 1649–1660. [Google Scholar] [CrossRef]
- Choi, D.G.; Venkatesan, J.; Shim, M.S. Selective Anticancer Therapy Using Pro-Oxidant Drug-Loaded Chitosan—Fucoidan Nanoparticles. Int. J. Mol. Sci. 2019, 20, 3220. [Google Scholar] [CrossRef]
- Tatini, F.; Landini, I.; Scaletti, F.; Massai, L.; Centi, S.; Ratto, F.; Nobili, S.; Romano, G.; Fusi, F.; Messori, L.; et al. Size Dependent Biological Profiles of PEGylated Gold Nanorods. J. Mater. Chem. B 2014, 2, 6072–6080. [Google Scholar] [CrossRef]
- De Clercq, E. Highlights in Antiviral Drug Research: Antivirals at the Horizon. Med. Res. Rev. 2013, 33, 1215–1248. [Google Scholar] [CrossRef]
- Guidi, F.; Landini, I.; Puglia, M.; Magherini, F.; Gabbiani, C.; Cinellu, M.A.; Nobili, S.; Fiaschi, T.; Bini, L.; Mini, E.; et al. Proteomic Analysis of Ovarian Cancer Cell Responses to Cytotoxic Gold Compounds. Metallomics 2012, 4, 307–314. [Google Scholar] [CrossRef]
- Magherini, F.; Fiaschi, T.; Valocchia, E.; Becatti, M.; Pratesi, A.; Marzo, T.; Massai, L.; Gabbiani, C.; Landini, I.; Nobili, S.; et al. Antiproliferative Effects of Two Gold(I)-N-Heterocyclic Carbene Complexes in A2780 Human Ovarian Cancer Cells: A Comparative Proteomic Study. Oncotarget 2018, 9, 28042–28068. [Google Scholar] [CrossRef]
- Mügge, C.; Rothenburger, C.; Beyer, A.; Görls, H.; Gabbiani, C.; Casini, A.; Michelucci, E.; Landini, I.; Nobili, S.; Mini, E.; et al. Structure, Solution Chemistry, Antiproliferative Actions and Protein Binding Properties of Non-Conventional Platinum(ii) Compounds with Sulfur and Phosphorus Donors. Dalton Trans. 2011, 40, 2006–2016. [Google Scholar] [CrossRef]
- Marzo, T.; Massai, L.; Pratesi, A.; Stefanini, M.; Cirri, D.; Magherini, F.; Becatti, M.; Landini, I.; Nobili, S.; Mini, E.; et al. Replacement of the Thiosugar of Auranofin with Iodide Enhances the Anticancer Potency in a Mouse Model of Ovarian Cancer. ACS Med. Chem. Lett. 2019, 10, 656–660. [Google Scholar] [CrossRef]
- Maiore, L.; Cinellu, M.A.; Nobili, S.; Landini, I.; Mini, E.; Gabbiani, C.; Messori, L. Gold(III) Complexes with 2-Substituted Pyridines as Experimental Anticancer Agents: Solution Behavior, Reactions with Model Proteins, Antiproliferative Properties. J. Inorg. Biochem. 2012, 108, 123–127. [Google Scholar] [CrossRef]
- Maiore, L.; Cinellu, M.A.; Michelucci, E.; Moneti, G.; Nobili, S.; Landini, I.; Mini, E.; Guerri, A.; Gabbiani, C.; Messori, L. Structural and Solution Chemistry, Protein Binding and Antiproliferative Profiles of Gold(I)/(III) Complexes Bearing the Saccharinato Ligand. J. Inorg. Biochem. 2011, 105, 348–355. [Google Scholar] [CrossRef]
- Landini, I.; Lapucci, A.; Pratesi, A.; Massai, L.; Napoli, C.; Perrone, G.; Pinzani, P.; Messori, L.; Mini, E. Selection and Characterization of a Human Ovarian Cancer Cell Line Resistant to Auranofin. Oncotarget 2017, 8, 96062–96078. [Google Scholar] [CrossRef]
- Guidi, F.; Modesti, A.; Landini, I.; Nobili, S.; Mini, E.; Bini, L.; Puglia, M.; Casini, A.; Dyson, P.J.; Gabbiani, C.; et al. The Molecular Mechanisms of Antimetastatic Ruthenium Compounds Explored through DIGE Proteomics. J. Inorg. Biochem. 2013, 118, 94–99. [Google Scholar] [CrossRef]
- Gamberi, T.; Massai, L.; Magherini, F.; Landini, I.; Fiaschi, T.; Scaletti, F.; Gabbiani, C.; Bianchi, L.; Bini, L.; Nobili, S.; et al. ScienceDirect Proteomic Analysis of A2780/S Ovarian Cancer Cell Response to the Cytotoxic Organogold (III) Compound Aubipy C. J. Proteom. 2014, 103, 103–120. [Google Scholar] [CrossRef]
- Sharifi, M.; Avadi, M.R.; Attar, F.; Dashtestani, F.; Ghorchian, H.; Rezayat, S.M.; Saboury, A.A.; Falahati, M. Cancer diagnosis using nanomaterials based electrochemical nanobiosensors. Biosens. Bioelectron. 2019, 126, 773–784. [Google Scholar] [CrossRef]
- Ambrosi, A.; Airò, F.; Merkoçi, A. Enhanced Gold Nanoparticle Based ELISA for a Breast Cancer Biomarker. Anal. Chem. 2010, 82, 1151–1156. [Google Scholar] [CrossRef]
- Ramos, M.; Castillo, C. Aplicaciones biomédicas de las nanopartículas magnéticas. Ide@s CONCYTEG 2011, 6, 629–646. [Google Scholar]
- Haun, J.B.; Yoon, T.J.; Lee, H.; Weissleder, R. Magnetic nanoparticle biosensors. Nanomed. Nanobiotechnol. 2010, 2, 291–304. [Google Scholar] [CrossRef]
- Riley, R.S.; June, C.H. Delivery Technologies for Cancer Immunotherapy. Nat. Rev. Drug Discov. 2013, 18, 175–196. [Google Scholar] [CrossRef]
- Park, W.; Heo, Y.; Han, D.K. New Opportunities for Nanoparticles in Cancer Immunotherapy. Biomater. Res. 2018, 22, 24. [Google Scholar] [CrossRef]
- Nam, J.; Son, S.; Park, K.S.; Zou, W.; Shea, L.D.; Moon, J.J. Cancer Nanomedicine for Combination Cancer Immunotherapy. Nat. Rev. Mater. 2019, 4, 398–414. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, W.; Shi, J.; Chen, N.; Fan, C. Nanoscale Delivery Systems for Cancer Immunotherapy. Mater. Horiz. 2018, 5, 344–362. [Google Scholar] [CrossRef]
- Mi, Y.; Smith, C.C.; Yang, F.; Qi, Y.; Roche, K.C.; Serody, J.S.; Vincent, B.G.; Wang, A.Z. A Dual Immunotherapy Nanoparticle Improves T-Cell Activation and Cancer Immunotherapy. Adv. Mater. 2018, 30, 1706098. [Google Scholar] [CrossRef]
- Surendran, S.P.; Moon, M.J.; Park, R.; Jeong, Y.Y. Bioactive Nanoparticles for Cancer Immunotherapy. Int. J. Mol. Sci. 2019, 19, 3877. [Google Scholar] [CrossRef]
- Toy, R.; Roy, K. Engineering Nanoparticles to Overcome Barriers to Immunotherapy. Bioeng. Transl. Med. 2016, 1, 47–62. [Google Scholar] [CrossRef]
- Mehadi, M.; Chowdhury, H.; Kubra, K.; Kanwar, R.K.; Kanwar, J.R. Nanoparticles Advancing Cancer Immunotherapy. In Biomedical Applications of Graphene and 2D Nanomaterials; Nurunnabi, M., McCarthy, J.R., Eds.; Elsevier: Cambridge, MA, USA, 2019; Chapter 13; pp. 283–304. [Google Scholar]
- Cheng, C.; Castro, G.; Liu, C.; Lau, P. Advanced Nanotechnology: An Arsenal to Enhance Immunotherapy in Fi Ghting Cancer. Clin. Chim. Acta 2019, 492, 12–19. [Google Scholar] [CrossRef]
- Hagan, C.T.; Medik, Y.B.; Wang, A.Z. Nanotechnology Approaches to Improving Cancer Immunotherapy. In Advances in Cancer Research; Academic Press: Cambridge, MA, USA, 2018; Volume 139, pp. 35–56. [Google Scholar]
- Rosalia, R.A.; Cruz, L.J.; Van Duikeren, S.; Tromp, A.T.; Oostendorp, J.; Silva, A.L.; Jiskoot, W.; De Gruijl, T.; Clemens, L.; Van Der Burg, S.H.; et al. CD40-Targeted Dendritic Cell Delivery of PLGA-Nanoparticle Vaccines Induce Potent Anti-Tumor Responses. Biomaterials 2014, 40, 88–97. [Google Scholar] [CrossRef]
- Nakamura, T.; Harashima, H. Integration of Nano Drug-Delivery System with Cancer Immunotherapy. Ther. Deliv. 2017, 8, 987–1000. [Google Scholar] [CrossRef]
- Latterich, M.; Corbeil, J. Label-free detection of biomolecular interactions in real time with a nano-porous silicon-based detection method. Proteome Sci. 2008, 6, 31. [Google Scholar]
- Jia, L.; Lu, Y.; Shao, J.; Liang, X.; Xu, Y. Nanoproteomics: A New Sprout from Emerging Links between Nanotechnology and Proteomics. Trends Biotechnol. 2013, 31, 99–107. [Google Scholar] [CrossRef]





| Applications | Findings | Conclusions | References |
|---|---|---|---|
| Tissue and implants engineering | Gold and titanium dioxide nanoparticles have been used to enhance cell proliferation rates for bone and cardiac tissue TiO2 nanoparticles conjugated with the polymer poly(lactic-co-glycolic acid) (PLGA), decrease harmful effects, match the nanostructured roughness of bone, and improve their cell performance. Nanofibers that serve as a peptide scaffold allow the regeneration of the axonal tissue. | Nanotechnology in tissue engineering is used to create, repair, and/or replace cells, tissues, and organs combining cells with bio-nanomaterials, and to provide the best micro-environment where cells must grow. Nano-scaffolds are used in tissue and implants engineering to regenerate central nervous system cells and possibly other organs. | [87,88,89] |
| Antimicrobial vehicules | Silver and titanium dioxide nanoparticles have antimicrobial properties that allow them to be used in surgical mask coatings by eliminating bacteria and viruses. | Drug coated nanoparticles have shown the potential to repel microorganisms and to act as a prevention tool. A unique property of nanomaterials is their high surface-to-volume ratio. Therefore, minuscule amounts of nanoparticles can lend substantial antimicrobial effects. | [90,91,92] |
| Gene delivery | Silica nanospheres functionalized with ammonium cation groups allow transfecting cell lipids, polymers, graphene, carbon nanotubes, nanospheres, and different types of inorganic particles to be used. | Nanoparticles have a great potential as vectors to deliver genetic material into living cells. | [93,94,95] |
| Cell separation | Magnetic nanoparticles (MNPs) allow magnetic bio-separations with low toxicity and high biocompatibility. At physiological pH and high salt concentrations, nanocomposites acquire a positive charge for easy electrostatic interactions. In general, the magnetic bio-separation of targeted biomolecules occurs thanks to the interaction between MNPs and a targeted molecule with a magnetic force. | Magnetic nanoparticles (MNPs) can be employed to separate biomolecules such as proteins, deoxyribonucleic acid (DNA), cells, bacteria, genes, and viruses depending on the specific functionalization of MNPs. | [95,96] |
| Biofuels | The use of Fe (0) nanoparticles favors the activity of bio-hydrogen production under anaerobic conditions. | Nanoparticles are attractive materials to produce sustainable energy resources, mainly biofuels, thanks to their large surface/volume ratio, which provides a greater number of active sites where they catalyze bio-hydrogen, biogas, biodiesel, and bioethanol production in a high yield. | [97,98,99] |
| Drug Delivery System (DDS) | A platinum derivate of a bile acid conjugated with multifunctional polymer-coated bio-ferrofluids as anti-tumor agent in osteosarcoma (MG-63) and T-cell leukemic (Jurkat) cells. The use of gold nanoparticles, polymer nanoparticles, or liposomes, among others, as excellent tumor peptide vaccine carriers play an important role in anti-tumor immunotherapy. | Nanoparticles-based drug delivery system (DDS) have been in the core of attention due to their unique and superior properties. These systems can enhance therapeutic efficacy by producing more favorable bio-availability, serum stability, and pharmacokinetics. Nanoparticle formulations provide better penetration and allow slow and controlled release of drug molecules at the target site for bioactivity | [100,101,102,103] |
| Anti-cancer chemotherapy | Chemical analogues with platinum (II)-based drugs or ruthenium-based antimetastatic agents have anti-cancer properties. The behavior and the biological properties of novel gold compounds containing different ligands have been reported for human ovarian cancer cells. One of the most studied gold (III) compounds is Auranofinan orally effective anti-rheumatic administered drug and an anti-cancer treatment. | Nanoparticles technology offers a series of advantages for drug delivery such as high loading yield, combination therapy, controlled release, prolonged circulation, and targeted delivery. Recently, platinum (II), ruthenium, and gold (III) compounds-based anti-cancer chemotherapy has been reported to kill cancer cells. Most of these studies have been done using proteomics approaches. | [49,104,105,106,107,108,109,110,111,112,113,114,115] |
| Biosensors | An enzyme-linked immunosorbent assay (ELISA) was developed in which nanoparticles (AuNPs) were used as carriers of the signalling antibody, anti-CA15-3-HRP, for the analysis of CA15-3, which is an important tumour marker useful for the follow-up of breast cancer. The use of magnetic nanoparticles as proximity sensors in magnetic resonance (NMR) is known as diagnostic magnetic resonance (DMR). | AuNPs can be used to improve the performance of studies, such as the classical ELISA test, which achieves greater sensitivities. The idea of using nanomaterials in biosensors arose from the possibility of lowering the detection limit (LOD) and improving the signal-to-noise ratio. A diagnostic magnetic resonance (DMR) is a powerful biosensor technology that offers advantages over other detection techniques as well as broad applicability for profiling different types of targets (DNA, proteins, metabolites, and cells). | [116,117,118,119] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auría-Soro, C.; Nesma, T.; Juanes-Velasco, P.; Landeira-Viñuela, A.; Fidalgo-Gomez, H.; Acebes-Fernandez, V.; Gongora, R.; Almendral Parra, M.J.; Manzano-Roman, R.; Fuentes, M. Interactions of Nanoparticles and Biosystems: Microenvironment of Nanoparticles and Biomolecules in Nanomedicine. Nanomaterials 2019, 9, 1365. https://doi.org/10.3390/nano9101365
Auría-Soro C, Nesma T, Juanes-Velasco P, Landeira-Viñuela A, Fidalgo-Gomez H, Acebes-Fernandez V, Gongora R, Almendral Parra MJ, Manzano-Roman R, Fuentes M. Interactions of Nanoparticles and Biosystems: Microenvironment of Nanoparticles and Biomolecules in Nanomedicine. Nanomaterials. 2019; 9(10):1365. https://doi.org/10.3390/nano9101365
Chicago/Turabian StyleAuría-Soro, Carlota, Tabata Nesma, Pablo Juanes-Velasco, Alicia Landeira-Viñuela, Helena Fidalgo-Gomez, Vanessa Acebes-Fernandez, Rafael Gongora, María Jesus Almendral Parra, Raúl Manzano-Roman, and Manuel Fuentes. 2019. "Interactions of Nanoparticles and Biosystems: Microenvironment of Nanoparticles and Biomolecules in Nanomedicine" Nanomaterials 9, no. 10: 1365. https://doi.org/10.3390/nano9101365
APA StyleAuría-Soro, C., Nesma, T., Juanes-Velasco, P., Landeira-Viñuela, A., Fidalgo-Gomez, H., Acebes-Fernandez, V., Gongora, R., Almendral Parra, M. J., Manzano-Roman, R., & Fuentes, M. (2019). Interactions of Nanoparticles and Biosystems: Microenvironment of Nanoparticles and Biomolecules in Nanomedicine. Nanomaterials, 9(10), 1365. https://doi.org/10.3390/nano9101365