Ionic Liquid-Nanostructured Poly(Methyl Methacrylate)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization Methods
2.2. Processing of IL-Modified PMMA
3. Results & Discussion
3.1. PMMA/IL Interactions
3.2. Consequences of the Presence of ILs on the Thermal Stability of PMMA
3.2.1. Thermal Stability of ILs
3.2.2. Thermal Stability of IL-Modified PMMA Materials
3.3. Surface Properties of IL-Modified PMMA
3.4. Mechanical Properties of IL-Modified PMMA
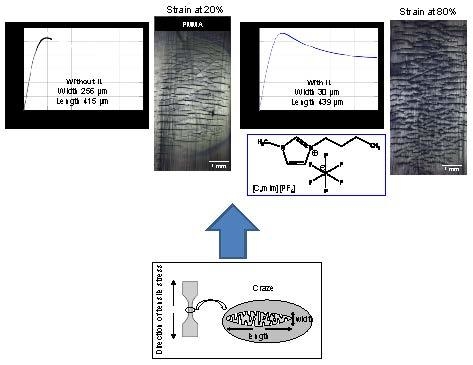
3.5. Influence of ILs on the Deformation Phenomena of IL-Modified PMMA
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Agrawal, S.; Patidar, D.; Dixit, M.; Sharma, K.; Saxena, N.S.; Pratap, A.; Saxena, N.S. Investigation of Thermo-Mechanical Properties of PMMA. AIP Conf. Proc. 2010, 79, 79–82. [Google Scholar]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Carraher, C.E., Jr. Free Radical Chain Polymerization: Addition Polymerization. In Introduction to Polymer Chemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- DiMaio, F.R. The science of bone cement: A historical review. Orthopedics 2002, 25, 1399–1407. [Google Scholar] [PubMed]
- Pawar, E. A Review Article on Acrylic PMMA. IOSR J. Mech. Civ. Eng. 2016, 13, 1–4. [Google Scholar]
- Higgs, W.A.; Lucksanasombool, P.; Higgs, R.J.E.D.; Swain, M.V. Comparison of the material properties of PMMA and glass ionomer based cements for use in orthopedic surgery. J. Mater. Sci. Mater. Med. 2001, 12, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Frazer, R.Q.; Byron, R.T.; Osborne, P.B.; West, K.P. PMMA: An essential material in medicine and dentistry. J. Long. Term. Eff. Med. Implant. 2005, 15, 629–639. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review, Compos. Sci. Technol. 2001, 61, 1189. [Google Scholar] [CrossRef]
- Ree, S.H.; Choi, J.Y. Synthesis of a Bioactive Poly (Methyl Methacrylate)/Silica Hybrid. Key Eng. Mater. 2002, 218, 433–436. [Google Scholar]
- Tjong, S.E. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng. R Rep. 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Larson, W.R.; Dixon, D.L.; Aquilino, S.A.; Clancy, J.M. The effect of carbon graphite fiber reinforcentent on the strength of provisional crown and fixed partial denture resins. J. Prosthet. Dent. 1991, 66, 816–820. [Google Scholar] [CrossRef]
- Kim, K.S.; Byun, J.H.; Lee, G.H.; Park, S.J. Influence of GMA grafted MWNTs on physical and rheological properties of PMMA-based nanocomposites by in situ polymerization. Macromol. Res. 2011, 19, 14–20. [Google Scholar] [CrossRef]
- Wu, W.; He, T.; Chen, J.; Zhang, X.; Chen, Y. Study on in situ preparation of nano calcium carbonate/PMMA composite particles. Mater. Lett. 2006, 60, 2410–2415. [Google Scholar] [CrossRef]
- Nikolaidis, A.K.; Achilias, D.S.; Karayannidis, G.P. Synthesis and characterization of PMMA/organomodified montmorillonite nanocomposites prepared by in situ bulk polymerization. Ind. Eng. Chem. Res. 2011, 50, 571–579. [Google Scholar] [CrossRef]
- Saladino, M.L.; Motaung, T.E.; Luyt, A.S.; Spinella, A.; Nasillo, G.; Caponetti, E. The effect of silica nanoparticles on the morphology, mechanical properties and thermal degradation kinetics of PMMA. Polym. Degrad. Stab. 2012, 97, 452–459. [Google Scholar] [CrossRef]
- Etienne, S.; Becker, C.; Ruch, D.; Grignard, B.; Cartigny, G.; Detrembleur, C.; Calberg, C.; Jerome, R. Effects of incorporation of modified silica nanoparticles on the mechanical and thermal properties of PMMA. J. Therm. Anal. Calorim. 2007, 87, 101–104. [Google Scholar] [CrossRef]
- Jayasuriya, M.M.; Hourston, D.J. The Effect of Composition and the Level of Crosslinking of the Poly(methylmethacrylate) Phase on the Properties of Natural Rubber-Poly(methylmethacrylate) Semi-2 Interpenetrating Polymer Networks. J. Appl. Polym. Sci. 2012, 124, 3558–3564. [Google Scholar] [CrossRef]
- Gutteridge, D.L. Reinforcement of poly (methyl methacrylate) with ultra-high-modulus polyethylene fibre. J. Dent. 1992, 20, 50–54. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, L.; Chen, G. Plasticizer-assisted bonding of Poly (Methyl methacrylate) microfluidic chips at low temperature. J. Chromatogr. A 2010, 1217, 160–166. [Google Scholar] [CrossRef]
- Flora, X.H. Role of Different Plasticizers in Li-Ion Conducting Poly (Acrylonitrile)-Poly (Methyl Methacrylate) Hybrid Polymer Electrolyte. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 37–41. [Google Scholar] [CrossRef]
- Garcia, N.; Corrales, T.; Guzman, J.; Tiemblo, P. Understanding the role of nanosilica particle surfaces in the thermal degradation of nanosilica–poly (methyl methacrylate) solution-blended nanocomposites: From low to high silica concentration. Polym. Degrad. Stab. 2007, 92, 635–643. [Google Scholar] [CrossRef]
- Chau, J.L.H.; Hsieh, C.C.; Lin, Y.M.; Li, A.K. Preparation of transparent silica–PMMA nanocomposite hard coatings. Prog. Org. Coat. 2008, 62, 436. [Google Scholar] [CrossRef]
- Lach, R.; Kim, G.M.; Michler, G.H.; Grellmann, W.; Albrecht, K. Indentation Fracture Mechanics for Toughness Assessment of PMMA/SiO2 Nanocomposites. Mater. Mater. Eng. 2006, 291, 263–271. [Google Scholar]
- Chau, J.L.H.; Hsieh, C.C.; Lin, Y.M.; Li, A.K. Effect of nanoparticle size and size-distribution on mechanical behavior of filled amorphous thermoplastic polymers. J. Appl. Polym. Sci. 2007, 105, 2577–2587. [Google Scholar]
- Livi, S.; Duchet-Rumeau, J.; Gérard, J.F.; Pham, T.N. Polymers and ionic liquids: A successful wedding. Macromol. Chem. Phys. 2015, 216, 359–368. [Google Scholar] [CrossRef]
- Ramesh, S.; Liew, C.W.; Ramesh, K. Evaluation and investigation on the effect of ionic liquid onto PMMA-PVC gel polymer blend electrolytes. J. Non. Cryst. Solids. 2011, 357, 2132–2138. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Daik, R. Applications of ionic liquids and their mixtures for preparation of advanced polymer blends and composites: A. short review. Rev. Adv. Mater. Sci. 2015, 40, 45–59. [Google Scholar]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem Soc Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Lins, L.C.; Livi, S.; Duchet-Rumeau, J.; Gérard, J.F. Phosphonium ionic liquids as new compatibilizing agents of biopolymer blends composed of poly (butylene-adipate-co-terephtalate)/poly (lactic acid) (PBAT/PLA). RSC Adv. 2015, 5, 59082–59092. [Google Scholar] [CrossRef]
- Stefanescu, C.; Daly, W.H.; Negulescu, I.I. Biocomposite films prepared from ionic liquid solutions of chitosan and cellulose. Carbohydr. Polym. 2012, 87, 435–443. [Google Scholar] [CrossRef]
- Yousfi, M.; Livi, S.; Duchet-Rumeau, J. Ionic liquids: A new way for the compatibilization of thermoplastic blends. Chem. Eng. J. 2014, 255, 513–524. [Google Scholar] [CrossRef]
- Livi, S.; Bugatti, V.; Marechal, M.; Soares, B.G.; Duchet-Rumeau, J.; Barra, G.M.O.; Gérard, J.F. Ionic Liquids-Lignin combination: An innovative way to improve mechanical behaviour and water vapour permeability of eco-designed biodegradable polymer blends. RSC Adv. 2015, 5, 1989–1998. [Google Scholar] [CrossRef]
- Yang, J.; Pruvost, S.; Livi, S.; Duchet-Rumeau, J. Understanding of Versatile and Tunable Nanostructuration of Ionic Liquids on Fluorinated Copolymer. Macromolecules 2015, 48, 4581–4590. [Google Scholar] [CrossRef]
- Lins, L.C.; Livi, S.; Maréchal, M.; Duchet-Rumeau, J.; Gerard, J.F. Structural dependence of cations and anions to building the polar phase of PVDF. Eur. Pol. J. 2018, 107, 236–248. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Livi, S.; Soares, B.G.; Pruvost, S.; Duchet-Rumeau, J.; Gérard, J.F. Ionic liquids: A New Route for the Design of Epoxy Networks. ACS Sustain. Chem. Eng. 2006, 4, 481–490. [Google Scholar] [CrossRef]
- Soares, B.G.; Riany, N.; Silva, A.A.; Barra, G.M.O.; Livi, S. Dual-role of phosphonium–based ionic liquid in epoxy/MWCNT systems: Electric, rheological behavior and electromagnetic interference shielding effectiveness. Eur. Polym. J. 2016, 84, 77–88. [Google Scholar] [CrossRef]
- Rahman, M.; Brazel, C.S. Ionic liquids: New generation stable plasticizers for poly (vinyl chloride). Polym. Degrad. Stab. 2006, 91, 3371–3382. [Google Scholar] [CrossRef]
- Sankri, A.; Arhaliass, A.; Dez, I.; Gaumont, A.C.; Grohens, Y.; Lourdin, D.; Pillin, I.; Rolland-Sabaté, A.; Leroy, E. Thermoplastic starch plasticized by an ionic liquid. Carbohydr. Polym. 2010, 82, 256–263. [Google Scholar] [CrossRef]
- Lins, L.C.; Bugatti, V.; Livi, S.; Gorrasi, G. Phosphonium ionic liquid as interfacial agent of layered double hydroxide: Application to a pectin matrix. Carbohydr. Polym. 2018, 182, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Lins, L.C.; Bugatti, V.; Livi, S.; Gorrasi, G. Ionic Liquid as Surfactant Agent of Hydrotalcite: Influence on the Final Properties of Polycaprolactone Matrix. Polymers 2018, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.M.A.; Marceneiro, S.; Braga, M.E.M.; Coelho, J.F.J.; Ferreira, A.G.M.; Simões, P.N.; Veiga, H.I.M.; Tomé, L.C.; Marrucho, I.M.; Esperança, J.M.S.S.; et al. Phosphonium-based ionic liquids as modifiers for biomedical grade poly (vinyl chloride). Acta Biomater. 2012, 8, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.K.; Wu, T.Y.; Chang, Y.M.; Chen, A.F. Ductile polylactic acid prepared with ionic liquids. Chem. Eng. J. 2013, 215, 886–893. [Google Scholar] [CrossRef]
- Scott, M.P.; Brazel, C.S.; Benton, M.G.; Mays, J.W.; Holbrey, D.; Rogers, R.D. Application of ionic liquids as plasticizers for poly (methyl methacrylate). Chem. Commun. 2002, 13, 1370–1371. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.P.; Rahman, M.; Brazel, C.S. Application of ionic liquids as low-volatility plasticizers for PMMA. Eur. Polym. J. 2003, 39, 1947–1953. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Ozawa, T. Estimation of activation energy by isoconversion methods. Thermochim. Acta 1992, 203, 159–165. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Livi, S.; Gérard, J.F.; Duchet-Rumeau, J. Ionic liquids: Structuration agents in a fluorinated matrix. Chem. Commun. 2011, 47, 3589–3591. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Miki, K.; Mukai, T.; Nishikawa, K.; Koga, Y. Hydrophobicity/hydrophilicity of 1-butyl-2, 3-dimethyl and 1-ethyl-3-methylimodazolium ions: toward characterization of room temperature ionic liquids. J. Phys. Chem. B 2009, 44, 14754–14760. [Google Scholar] [CrossRef] [PubMed]
- Vitucci, F.M.; Trequattrini, F.; Palumbo, O.; Brubach, J.B.; Roy, P.; Paolone, A. Infrared spectra of bis(trifluoromethanesulfonyl)imide based ionic liquids: Experiments and DFT simulations. Vib. Spectrosc. 2014, 74, 81–87. [Google Scholar] [CrossRef]
- Herstedt, M.; Smirnov, M.; Johansson, P.; Chami, M.; Grondin, J.; Servant, L.; Lassègues, J.C. Spectroscopic characterization of the conformational states of the bis(trifluoromethanesulfonyl)imide anion (TFSI). J. Raman Spectrosc. 2005, 36, 762–770. [Google Scholar] [CrossRef]
- Ueno, K.; Fukai, T.; Nagatsuka, T.; Yasuda, T.; Watanabe, M. Solubility of poly (methyl methacrylate) in ionic liquids in relation to solvent parameters. Langmuir 2014, 30, 3228–3235. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.L.S.; Neves, C.M.S.S.; Carvalho, P.J.; Gani, R.; Coutinho, J.A.P. Chameleonic behavior of ionic liquids and its impact on the estimation of solubility parameters. J. Phys. Chem. B 2011, 115, 12879–12888. [Google Scholar] [CrossRef] [PubMed]
- Vitucci, F.M.; Trequattrini, F.; Palumbo, O.; Brubach, J.B.; Roy, P.; Navarra, M.A.; Panero, S.; Paolone, A. Stabilization of Different Conformers of Bis(trifluoromethanesulfonyl)imide Anion in Ammonium-Based Ionic Liquids at Low Temperatures. J. Phys. Chem. A 2014, 118, 8758–8764. [Google Scholar] [CrossRef]
- Livi, S.; Gérard, J.F.; Duchet-Rumeau, J. Ionic Liquids as Polymer Additives. In Applications of Ionic Liquids in Polymer Science Technology; Mecerreyes, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–21. [Google Scholar]
- Maton, C.; de Vos, N.; Stevens, C.V. Ionic liquid thermal stabilities: Decomposition mechanisms and analysis tools. Chem. Soc. Rev. 2016, 42, 5963–5977. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Chan, B.K.M.; Chang, N.H.; Grimmett, M.R. The Synthesis and Thermolysis of Imidazole Quaternary Salts. Aust. J. Chem. 1977, 30, 2005–2013. [Google Scholar] [CrossRef]
- Gordon, J.E. Fused Organic Salts. III. 1a Chemical Stability of Molten Tetra-n-alkylammonium Salts. Medium Effects on Thermal R4N+X- Decomposition. RBr + I- = RI + Br- Equilibrium Constant in Fused Salt Medium. J. Org. Chem. 1965, 30, 2760–2763. [Google Scholar] [CrossRef]
- Kamavaram, V.; Reddy, R.G. Thermal stabilities of di-alkylimidazolium chloride ionic liquids. Int. J. Therm. Sci. 2008, 47, 773–777. [Google Scholar] [CrossRef]
- Awad, W.H.; Gilman, J.W.; Nyden, M.; Harris, R.H.; Sutto, T.E.; Callahan, J.; Trulove, P.C.; DeLong, H.C.; Fox, D.M. Thermal degradation studies of alkyl-imidazolium salts and their application in nanocomposites. Thermochim. Acta 2004, 409, 3–11. [Google Scholar] [CrossRef]
- Ngo, H.L.; LeCompte, K.; Hargens, L.; McEwen, A.B. Thermal properties of imidazolium ionic liquids. Thermochim. Acta 2000, 357, 97–102. [Google Scholar] [CrossRef]
- Baranyai, K.J.; Deacon, G.B.; MacFarlane, D.R.; Pringle, J.M.; Scott, J.L. Thermal degradation of ionic liquids at elevated temperatures. Aust. J. Chem. 2004, 57, 145–147. [Google Scholar] [CrossRef]
- Begg, C.G.; Grimmett, M.R.; Wethey, P.D. The thermally induced rearrangement of 1-substituted imidazoles. Aust. J. Chem. 1973, 26, 2435–2446. [Google Scholar] [CrossRef]
- Lethesh, K.C.; Dehaen, W.; Binnemans, K. Base stable quaternary ammonium ionic liquids. RSC Adv. 2014, 4, 4472–4477. [Google Scholar] [CrossRef]
- Kilaru, P.; Baker, G.A.; Scovazzo, P. Density and surface tension measurements of imidazolium-, quaternary phosphonium-, and ammonium-based room-temperature ionic liquids: Data and correlations. J. Chem. Eng. Data 2007, 52, 2306–2314. [Google Scholar] [CrossRef]
- Freire, M.G.; Carvalho, P.J.; Fernandes, A.M.; Marrucho, I.M.; Queimada, A.J.; Coutinho, J.A.P. Surface tensions of imidazolium based ionic liquids: Anion, cation, temperature and water effect. J. Colloid Interface Sci. 2007, 314, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.M.N.B.F.; Lopes, J.N.C.; Esperança, J.M.S.S.; Gomes, L.R.; Marrucho, I.M.; Rebelo, L.P.N. Ionic Liquids: First Direct Determination of their Cohesive Energy. J. Am. Chem. Soc. 2007, 129, 284–285. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Nishikawa, K.; Koga, Y. Relative hydrophobicity and hydrophilicity of some “Ionic liquid” Anions determined by the 1-propanol probing methodology: A differential thermodynamic approach. J. Phys. Chem. B 2008, 112, 2655–2660. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, Y.; Cao, X.; You, J.; Dong, W. Multifunctional role of an ionic liquid in melt-blended poly (methyl methacrylate)/multi-walled carbon nanotube nanocomposites. Nanotechnology 2012, 23, 255702. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, P. The Effect of Concentration and Type of Plasticizer on the Mechanical Properties of Cellulose Acetate Butyrate Organic-Inorganic Hybrids. In Recent Advances in Plasticizers; Luqman, M., Ed.; InTech: Vienna, Austria, 2012; pp. 141–164. [Google Scholar]
- Luo, W.; Liu, W. Incubation time to crazing in stressed poly (methyl methacrylate). Polym. Test. 2007, 26, 413–418. [Google Scholar] [CrossRef]
- Andrews, E.H.; Bevan, L. Mechanics and mechanism of environmental crazing in a polymeric glass. Polymer 1972, 13, 337–346. [Google Scholar] [CrossRef]
- Bucknall, C.B. Role of surface chain mobility in crazing. Polymer 2012, 53, 4778–4786. [Google Scholar] [CrossRef]
- Scheirs, J. Compositional and Failure Analysis of Polymers: A Practical Approach, 1st ed.; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Plummer, C.J.G.; Donald, A.M. Crazing mechanisms and craze healing in glassy polymers. J. Mater. Sci. 1989, 24, 1399–1405. [Google Scholar] [CrossRef]
- Mahajan, D.K.; Hartmaier, A. Mechanisms of crazing in glassy polymers revealed by molecular dynamics simulations. Phys. Rev. E 2012, 86, 021802. [Google Scholar] [CrossRef] [PubMed]







| Designation | Cation | Anion | Tm (K) | Mm (g·mol−1) |
|---|---|---|---|---|
| (C4mIm)(PF6) | 1-butyl-3-methylimidazolium | Hexafluorophosphate | 283 | 284 |
| (N1,1,1,4)(PF6) | N-trimethyl-N-butylammoniun | Hexafluorophosphate | 393 | 289 |
| (N1,1,1,6)(Br) | N-trimethyl-N-hexylammonium | Bromide | 268 | 224 |
| (N1,1,1,6)(TFSI) | N-trimethyl-N-hexylammonium | Bis(trifluoromethanesulfonyl)imide | 308 | 424 |
| Material | Tg/K | Tonset/K | Tmax/K | Tfinal/K |
|---|---|---|---|---|
| PMMA | 367 | 614 | 656 | 691 |
| PMMA/(C4mIm)(PF6) | 360 | 627 | 667 | 708 |
| PMMA/(N1,1,1,4)(PF6) | 364 | 617 | 662 | 705 |
| PMMA/(N1,1,1,6)(Br) | 363 | 502 a/614 b | 548 a/659 b | 693 |
| PMMA/(N1,1,1,6)(TFSI) | 367 | 619 | 666 | 699 |
| Sample | θH2O (°) | θCH2I2 (°) | γt (mN·m−1) | γd (mN·m−1) | γnd (mN·m−1) |
|---|---|---|---|---|---|
| PMMA | 71 ± 1 | 40.1 ± 1.7 | 40.7 | 30.3 | 10.4 |
| PMMA/(C4mIm)(PF6) | 72 ± 1 | 27.0 ± 1.1 | 45.3 | 40.1 | 5.2 |
| PMMA/(N1,1,1,4)(PF6) | 72 ± 1 | 26.6 ± 1.7 | 45.3 | 40.5 | 4.8 |
| PMMA/(N1,1,1,6)(Br) | 66 ± 1 | 34.0 ± 0.9 | 44.6 | 35.1 | 9.5 |
| PMMA/(N1,1,1,6)(TFSI) | 73 ± 1 | 40 ± 0.7 | 43.0 | 37.8 | 5.2 |
| Material | Young’s modulus (MPa) | Strain at Break (%) |
|---|---|---|
| PMMA | 953 ± 7 | 24 ± 1 |
| PMMA/(C4mIm)(PF6) | 939 ± 2 | 96 ± 3 |
| PMMA/(N1,1,1,4)(PF6) | 943 ± 5 | 70 ± 4 |
| PMMA/(N1,1,1,6)(Br) | 920 ± 7 | 92 ± 3 |
| PMMA/(N1,1,1,6)(TFSI) | 934 ± 2 | 68 ± 4 |
| Strain (%) | Craze Width (μm) | Craze Length (μm) | Craze Density (crazes·mm−2) | |
|---|---|---|---|---|
| PMMA | 20 | 256 | 415 | 770 |
| PMMA/ (C4mIm)(PF6) | 40 | 18 | 332 | 1060 |
| 50 | 23 | 361 | 1030 | |
| 60 | 24 | 395 | 970 | |
| PMMA/ (N1,1,1,4)(PF6) | 40 | 22 | 416 | 490 |
| 50 | 30 | 499 | 520 | |
| 60 | 39 | 531 | 550 | |
| PMMA/ (N1,1,1,6)(TFSI) | 40 | 24 | 285 | 340 |
| 50 | 31 | 464 | 340 | |
| 60 | 39 | 535 | 350 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedosse Zornio, C.; Livi, S.; Duchet-Rumeau, J.; Gerard, J.-F. Ionic Liquid-Nanostructured Poly(Methyl Methacrylate). Nanomaterials 2019, 9, 1376. https://doi.org/10.3390/nano9101376
Fedosse Zornio C, Livi S, Duchet-Rumeau J, Gerard J-F. Ionic Liquid-Nanostructured Poly(Methyl Methacrylate). Nanomaterials. 2019; 9(10):1376. https://doi.org/10.3390/nano9101376
Chicago/Turabian StyleFedosse Zornio, Clarice, Sébastien Livi, Jannick Duchet-Rumeau, and Jean-François Gerard. 2019. "Ionic Liquid-Nanostructured Poly(Methyl Methacrylate)" Nanomaterials 9, no. 10: 1376. https://doi.org/10.3390/nano9101376
APA StyleFedosse Zornio, C., Livi, S., Duchet-Rumeau, J., & Gerard, J. -F. (2019). Ionic Liquid-Nanostructured Poly(Methyl Methacrylate). Nanomaterials, 9(10), 1376. https://doi.org/10.3390/nano9101376