A Review of Recent Advances of Dielectric Barrier Discharge Plasma in Catalysis
Abstract
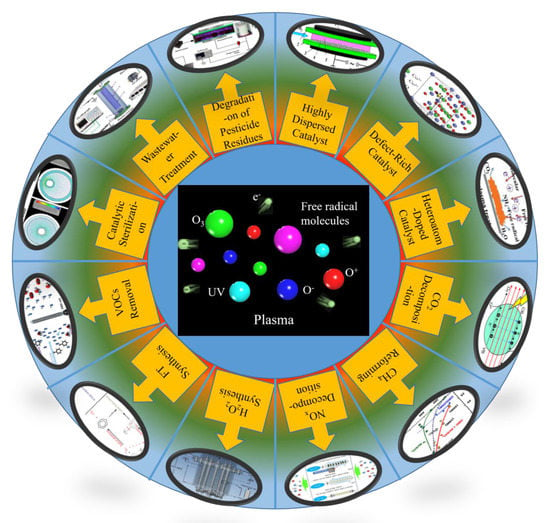
:1. Introduction
2. Preparation of Catalyst by DBD Plasma
2.1. Highly Dispersed Catalyst
2.1.1. Precious Metals
2.1.2. Non-Precious Metals
2.2. Defect-Rich Catalyst
2.2.1. Carbon Material Defect
2.2.2. Metal and Metal Oxide Defect
2.3. Heteroatom-Doped Catalyst
2.3.1. Nitrogen Atom Doping
2.3.2. Sulphur Atom Doping
2.3.3. Phosphorus Atom Doping
3. DBD Plasma Catalysis
3.1. Application of C1 Chemistry
3.1.1. Methanation
3.1.2. CO Oxidation
3.1.3. CO2 Decomposition
3.1.4. CH4 Reforming
3.2. NOx Decomposition
3.3. H2O2 Synthesis
3.4. Fischer–Tropsch Synthesis
3.5. Volatile Organic Compounds Removal
3.6. Catalytic Sterilization
3.7. Wastewater Treatment
3.7.1. Pharmaceutical Wastewater
3.7.2. Dyestuff Wastewater
3.7.3. Grease Wastewater
3.7.4. Antibiotic Wastewater
3.8. Degradation of Pesticide Residues
3.8.1. Insecticide
3.8.2. Herbicide
3.8.3. Germicide
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Snoeckx, R.; Bogaerts, A. Plasma technology—a novel solution for CO2 conversion? Chem. Soc. Rev. 2017, 46, 5805–5863. [Google Scholar] [CrossRef]
- Liao, X.; Liu, D.; Xiang, Q.; Ahn, J.; Chen, S.; Ye, X.; Ding, T. Inactivation mechanisms of non-thermal plasma on microbes: a review. Food Control 2017, 75, 83–91. [Google Scholar] [CrossRef]
- Kizling, M.B.; Järås, S.G. A review of the use of plasma techniques in catalyst preparation and catalytic reactions. Appl. Catal. A Gen. 1996, 147, 1–21. [Google Scholar] [CrossRef]
- Liu, C.J.; Vissokov, G.P.; Jang, B.W.L. Catalyst preparation using plasma technologies. Catal. Today 2002, 72, 173–184. [Google Scholar] [CrossRef]
- Siemens, W. Ueber die elektrostatische induction und die verzögerung des stroms in flaschendrähten. Annalen der Physik 1857, 178, 66–122. [Google Scholar] [CrossRef]
- Andrews, T.; Tait, P.G. VII. On the volumetric relations of ozone, and the action of the electrical discharge on oxygen and other gases. Philos. Trans. R. Soc. Lond. 1860, 150, 113–131. [Google Scholar]
- Kogelschatz, U. Dielectric-barrier discharges: their history, discharge physics, and industrial applications. Plasma Chem. Plasma Process. 2003, 23, 1–46. [Google Scholar] [CrossRef]
- Wagner, H.E.; Brandenburg, R.; Kozlov, K.; Sonnenfeld, A.; Michel, P.; Behnke, J. The barrier discharge: basicproperties and applications to surface treatment. Vacuum 2003, 71, 417–436. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, G.; Liu, X.; Phan, A.N.; Luo, K. A study on CO2 decomposition to CO and O2 by the combination of catalysis and dielectric-barrier discharges at low temperatures and ambient pressure. Ind. Eng. Chem. Res. 2017, 56, 3204–3216. [Google Scholar] [CrossRef]
- Liu, C.J.; Ye, J.; Jiang, J.; Pan, Y. Progresses in the preparation of coke resistant Ni-based catalyst for steam and CO2 reforming of methane. Chemcatchem 2015, 3, 529–541. [Google Scholar] [CrossRef]
- Neyts, E.C.; Ostrikov, K.; Sunkara, M.K.; Bogaerts, A. Plasma catalysis: synergistic effects at the nanoscale. Chem. Rev. 2016, 116, 767. [Google Scholar] [CrossRef]
- Kenward, M. Why plasma science means business. Phys. World 1995, 8, 31–34. [Google Scholar] [CrossRef]
- Liu, C.; Li, M.; Wang, J.; Zhou, X.; Guo, Q.; Yan, J.; Li, Y. Plasma methods for preparing green catalysts: current status and perspective. Chin. J. Catal. 2016, 37, 340–348. [Google Scholar] [CrossRef]
- Pinna, F. Supported metal catalysts preparation. Catal. Today 1998, 41, 129–137. [Google Scholar] [CrossRef]
- Mojet, B.; Miller, J.; Ramaker, D.; Koningsberger, D. A new model describing the metal–support interaction in noble metal catalysts. J. Catal. 1999, 186, 373–386. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, H.; Na, B.K.; Song, H.K. Plasma-assisted reduction of supported metal catalyst using atmospheric dielectric-barrier discharge. Catal. Today 2004, 89, 193–200. [Google Scholar] [CrossRef]
- Di, L.; Xu, Z.; Wang, K.; Zhang, X. A facile method for preparing Pt/TiO2 photocatalyst with enhanced activity using dielectric barrier discharge. Catal. Today 2013, 211, 109–113. [Google Scholar] [CrossRef]
- Di, L.; Zhang, X.; Xu, Z.; Wang, K. Atmospheric-pressure cold plasma for preparation of high performance Pt/TiO2 photocatalyst and its mechanism. Plasma Chem. Plasma Process. 2014, 34, 301–311. [Google Scholar] [CrossRef]
- Di, L.; Xu, Z.; Zhang, X. Atmospheric-pressure cold plasma for synthesizing Ag modified Degussa P25 with visible light activity using dielectric barrier discharge. Catal. Today 2013, 211, 143–146. [Google Scholar] [CrossRef]
- Xu, Z.; Qi, B.; Di, L.; Zhang, X. Partially crystallized Pd nanoparticles decorated TiO2 prepared by atmospheric-pressure cold plasma and its enhanced photocatalytic performance. J. Energy Chem. 2014, 23, 679–683. [Google Scholar] [CrossRef]
- Qi, B.; Di, L.; Xu, W.; Zhang, X. Dry plasma reduction to prepare a high performance Pd/C catalyst at atmospheric pressure for CO oxidation. J. Mater. Chem. A 2014, 2, 11885–11890. [Google Scholar] [CrossRef]
- Xu, W.; Zhan, Z.; Di, L.; Zhang, X. Enhanced activity for CO oxidation over Pd/Al2O3 catalysts prepared by atmospheric-pressure cold plasma. Catal. Today 2015, 256, 148–152. [Google Scholar] [CrossRef]
- Di, L.; Xu, W.; Zhan, Z.; Zhang, X. Synthesis of alumina supported Pd–Cu alloy nanoparticles for CO oxidation via a fast and facile method. RSC Adv. 2015, 5, 71854–71858. [Google Scholar] [CrossRef]
- Di, L.; Duan, D.; Park, D.W.; Ahn, W.S.; Lee, B.J.; Zhang, X. Cold plasma for synthesizing high performance bimetallic PdCu catalysts: effect of reduction sequence and Pd/Cu atomic ratios. Top. Catal. 2017, 60, 925–933. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Zhou, Q.; Meng, B.; Zhao, J.; Qiu, J.; Gogotsi, Y. Low-temperature plasma-assisted preparation of graphene supported palladium nanoparticles with high hydrodesulfurization activity. J. Mater. Chem. 2012, 22, 14363–14368. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.S.; Zhu, B.; Liu, J.L.; Zhu, X.; Zhu, A.M.; Jang, W.L. Atmospheric-pressure O2 plasma treatment of Au/TiO2 catalysts for CO oxidation. Catal. Today 2015, 256, 142–147. [Google Scholar] [CrossRef]
- Lanbo, D.; Zhibin, Z.; Xiuling, Z.; Bin, Q.; Weijie, X. Atmospheric-pressure DBD cold plasma for preparation of high active Au/P25 catalysts for low-temperature CO oxidation. Plasma Sci. Technol. 2016, 18, 544–548. [Google Scholar]
- Hua, W.; Jin, L.; He, X.; Liu, J.; Hu, H. Preparation of Ni/MgO catalyst for CO2 reforming of methane by dielectric-barrier discharge plasma. Catal. Commun. 2010, 11, 968–972. [Google Scholar] [CrossRef]
- Bian, L.; Zhang, L.; Zhu, Z.; Li, Z. Methanation of carbon oxides on Ni/Ce/SBA-15 pretreated with dielectric barrier discharge plasma. Mol. Catal. 2018, 446, 131–139. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, Z.; Yan, X.; Ma, X.; Hao, Q. CO methanation over Ni/SiO2 catalyst prepared by ammonia impregnation and plasma decomposition. Top. Catal. 2017, 60, 879–889. [Google Scholar] [CrossRef]
- Jia, X.; Rui, N.; Zhang, X.; Hu, X.; Liu, C.J. Ni/ZrO2 by dielectric barrier discharge plasma decomposition with improved activity and enhanced coke resistance for CO methanation. Catal. Today 2018, 334, 215–222. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Z.; Wang, Y. Ni/MgO catalyst prepared via dielectric-barrier discharge plasma with improved catalytic performance for carbon dioxide reforming of methane. Front. Chem. Sci. Eng. 2014, 8, 133–140. [Google Scholar] [CrossRef]
- Fan, Z.; Sun, K.; Rui, N.; Zhao, B.; Liu, C.J. Improved activity of Ni/MgAl2O4 for CO2 methanation by the plasma decomposition. J. Energy Chem. 2015, 24, 655–659. [Google Scholar] [CrossRef]
- Zhang, M.; Li, P.; Zhu, M.; Tian, Z.; Dan, J.; Li, J.; Dai, B.; Yu, F. Ultralow-weight loading Ni catalyst supported on two-dimensional vermiculite for carbon monoxide methanation. Chin. J. Chem. Eng. 2018, 26, 1873–1878. [Google Scholar] [CrossRef]
- Guo, X.; Sun, Y.; Yu, Y.; Zhu, X.; Liu, C.J. Carbon formation and steam reforming of methane on silica supported nickel catalysts. Catal. Commun. 2012, 19, 61–65. [Google Scholar] [CrossRef]
- Guo, Y.F.; Ye, D.Q.; Chen, K.F.; He, J.C.; Chen, W.L. Toluene decomposition using a wire-plate dielectric barrier discharge reactor with manganese oxide catalyst in situ. J. Mol. Catal. A-Chem. 2006, 245, 93–100. [Google Scholar] [CrossRef]
- Fu, T.; Huang, C.; Lv, J.; Li, Z. Fuel production through Fischer–Tropsch synthesis on carbon nanotubes supported Co catalyst prepared by plasma. Fuel 2014, 121, 225–231. [Google Scholar] [CrossRef]
- Dai, H.; Zhu, M.; Zhao, D.; Yu, F.; Dai, B. Effective catalytic performance of plasma-enhanced W2N/AC as catalysts for acetylene hydrochlorination. Top. Catal. 2017, 60, 1016–1023. [Google Scholar] [CrossRef]
- El-Roz, M.; Lakiss, L.; El, F.J.; Lebedev, O.I.; Thibault-Starzyk, F.; Valtchev, V. Incorporation of clusters of titanium oxide in Beta zeolite structure by a new cold TiCl4-plasma process: physicochemical properties and photocatalytic activity. Phys. Chem. Chem. Phys. 2013, 15, 16198–16207. [Google Scholar] [CrossRef]
- Di, L.; Zhang, J.; Zhang, X. A review on the recent progress, challenges, and perspectives of atmospheric-pressure cold plasma for preparation of supported metal catalysts. Plasma Process. Polym. 2018, 15, 1700234. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Huo, J.; Chen, R.; Dai, L.; Wang, S. Defect chemistry of nonprecious-metal electrocatalysts for oxygen reactions. Adv. Mater. 2017, 29, 1606459. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; He, J.; Liu, Q.; Yao, T.; Chen, L.; Yan, W.; Hu, F.; Jiang, Y.; Zhao, Y.; Hu, T. Vacancy-induced ferromagnetism of MoS2 nanosheets. J. Am. Chem. Soc. 2015, 137, 2622–2627. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.; Yan, D.Y.; Jiao, Y.; Wang, H.; Zheng, Y.; Zheng, X.; Mao, J.; Du, X.W.; Hu, Z.; Jaroniec, M. Engineering surface atomic structure of single-crystal cobalt (II) oxide nanorods for superior electrocatalysis. Nat. Commun. 2016, 7, 12876. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Han, Y.; Kong, X.; Deng, X.; Park, H.J.; Guo, Y.; Jin, S.; Qi, Z.; Lee, Z.; Qiao, Z. The origin of improved electrical double-layer capacitance by inclusion of topological defects and dopants in graphene for supercapacitors. Angew. Chem. 2016, 55, 13822–13827. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Meng, C.G.; Han, Y. Defective graphene supported MPd12 (M = Fe, Co, Ni, Cu, Zn, Pd) nanoparticles as potential oxygen reduction electrocatalysts: a first-principles study. J. Phys. Chem. C 2013, 117, 1350–1357. [Google Scholar] [CrossRef]
- Lai, L.; Potts, J.R.; Zhan, D.; Wang, L.; Poh, C.K.; Tang, C.; Gong, H.; Shen, Z.; Lin, J.; Ruoff, R.S. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy Environ. Sci. 2012, 5, 7936–7942. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, S.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X.; Wu, Q.; Ma, J.; Ma, Y.; Hu, Z. Boron-doped carbon nanotubes as metal-free electrocatalysts for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2011, 50, 7132–7135. [Google Scholar] [CrossRef]
- Chen, S.; Bi, J.; Zhao, Y.; Yang, L.; Zhang, C.; Ma, Y.; Wu, Q.; Wang, X.; Hu, Z. Nitrogen-doped carbon nanocages as efficient metal-free electrocatalysts for oxygen reduction reaction. Adv. Mater. 2012, 24, 5593–5597. [Google Scholar] [CrossRef]
- Silva, R.; Al-Sharab, J.; Asefa, T. Edge-plane-rich nitrogen-doped carbon nanoneedles and efficient metal-free electrocatalysts. Angew. Chem. Int. Ed. 2012, 51, 7171–7175. [Google Scholar] [CrossRef]
- Wang, W.H.; Huang, B.C.; Wang, L.S.; Ye, D.Q. Oxidative treatment of multi-wall carbon nanotubes with oxygen dielectric barrier discharge plasma. Surf. Coat. Technol. 2011, 205, 4896–4901. [Google Scholar] [CrossRef]
- Solís-Fernández, P.; Paredes, J.; López, M.J.; Cabria, I.; Alonso, J.A.; Martínez-Alonso, A.; Tascón, J. A combined experimental and theoretical investigation of atomic-scale defects produced on graphite surfaces by dielectric barrier discharge plasma treatment. J. Phys. Chem. C 2009, 113, 18719–18729. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J.; Wang, S.; Dai, L. Plasma-engraved Co3O4 nanosheets with oxygen vacancies and high surface area for the oxygen evolution reaction. Angew. Chem. Int. Ed. Engl. 2016, 55, 5277–5281. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.; Choi, Y.W.; Bagger, A.; Scholten, F.; Bonifacio, C.; Sinev, I.; Divins, N.J.; Zegkinoglou, I.; Jeon, H.S.; Kisslinger, K. Enhanced carbon dioxide electroreduction to carbon monoxide over defect rich plasma-activated silver catalysts. Angew. Chem. Int. Ed. 2017, 56, 11394–11398. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, W.; Wang, F.; Di, L.; Yang, S.; Zhu, S.; Yao, Y.; Ma, C.; Dai, B.; Yu, F. Enhanced photocatalytic degradation of organic dyes via defect-rich TiO2 prepared by dielectric barrier discharge plasma. Nanomaterials 2019, 9, 720. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, K.; Tanaka, T.; Ikegami, T.; Yamagata, Y.; Matsunaga, T.; Yamashita, K.; Oyama, Y. Application of the dielectric barrier discharge to detect defects in a teflon coated metal surface. J. Phys. D Appl. Phys. 2003, 36, 2883–2886. [Google Scholar] [CrossRef]
- Liu, X.; Dai, L. Carbon-based metal-free catalysts. Nat. Rev. Mater. 2016, 1, 16064. [Google Scholar] [CrossRef]
- Leem, J.H.; Lee, D.H.; Sang, Y.L. Properties of N-doped ZnO grown by DBD-PLD. Thin Solid Film. 2009, 518, 1238–1240. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, F.; Zhu, M.; Ma, C.; Zhao, D.; Wang, C.; Zhou, A.; Dai, B.; Ji, J.; Guo, X. N-doping of plasma exfoliated graphene oxide via dielectric barrier discharge plasma treatment for the oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 2011–2017. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Zhou, F.; Zhang, H.; Bai, J.; Wang, Y.; Wang, H. Preparation of N-vacancy-doped gC3N4 with outstanding photocatalytic H2O2 production ability by dielectric barrier discharge plasma treatment. Chin. J. Catal. 2018, 39, 1090–1098. [Google Scholar] [CrossRef]
- Luo, Z.; Jiang, H.; Li, D.; Hu, L.; Geng, W.; Wei, P.; Ouyang, P. Improved photocatalytic activity and mechanism of Cu2O/N–TiO2 prepared by a two-step method. RSC Adv. 2014, 4, 17797–17804. [Google Scholar] [CrossRef]
- Lin, M.; Hu, S.; Ping, L.; Wang, Q.; Ma, H.; Wei, L. In situ synthesis of sulfur doped carbon nitride with enhanced photocatalytic performance using DBD plasma treatment under H2S atmosphere. J. Phys. Chem. Solids 2018, 118, 166–171. [Google Scholar]
- Xiao, Z.H.; Wang, Y.; Huang, Y.C.; Wei, Z.X.; Dong, C.L.; Ma, J.M.; Shen, S.H.; Li, Y.F.; Wang, S.Y. Filling the oxygen vacancies in Co3O4 with phosphorus: an ultra-efficient electrocatalyst for overall water splitting. Energy Environ. Sci. 2017, 10, 2563–2569. [Google Scholar] [CrossRef]
- Neyts, E.C. Plasma-surface interactions in plasma catalysis. Plasma Chem. Plasma Process. 2016, 36, 185–212. [Google Scholar] [CrossRef]
- Chen, H.L.; Lee, H.M.; Chen, S.H.; Chao, Y.; Chang, M.B. Review of plasma catalysis on hydrocarbon reforming for hydrogen production—interaction, integration, and prospects. Appl. Catal. B Environ. 2008, 85, 1–9. [Google Scholar] [CrossRef]
- Van Durme, J.; Dewulf, J.; Leys, C.; Van Langenhove, H. Combining non-thermal plasma with heterogeneous catalysis in waste gas treatment: a review. Appl. Catal. B Environ. 2008, 78, 324–333. [Google Scholar] [CrossRef]
- Neyts, E.; Bogaerts, A. Understanding plasma catalysis through modelling and simulation—a review. J. Phys. D Appl. Phys. 2014, 47, 224010. [Google Scholar] [CrossRef]
- Vandenbroucke, A.M.; Morent, R.; De Geyter, N.; Leys, C. Non-thermal plasmas for non-catalytic and catalytic VOC abatement. J. Hazard. Mater. 2011, 195, 30–54. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yu, F.; Altaf, N.; Zhu, M.; Li, J.; Dai, B.; Wang, Q. Two-dimensional layered double hydroxides for reactions of methanation and methane reforming in C1 chemistry. Materials 2018, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- Mok, Y.S.; Kang, H.C.; Lee, H.J.; Koh, D.J.; Shin, D.N. Effect of nonthermal plasma on the methanation of carbon monoxide over nickel catalyst. Plasma Chem. Plasma Process. 2010, 30, 437–447. [Google Scholar] [CrossRef]
- Guo, X.; Traitangwong, A.; Hu, M.; Zuo, C.; Meeyoo, V.; Peng, Z.; Li, C. Carbon dioxide methanation over nickel-based catalysts supported on various mesoporous material. Energy Fuels 2018, 32, 3681–3689. [Google Scholar] [CrossRef]
- Li, P.; Wen, B.; Yu, F.; Zhu, M.; Guo, X.; Han, Y.; Kang, L.; Huang, X.; Dan, J.; Ouyang, F. High efficient nickel/vermiculite catalyst prepared via microwave irradiation-assisted synthesis for carbon monoxide methanation. Fuel 2016, 171, 263–269. [Google Scholar] [CrossRef]
- Song, Q.; Altaf, N.; Zhu, M.; Li, J.; Ren, X.; Dan, J.; Dai, B.; Louis, B.; Wang, Q.; Yu, F. Enhanced low-temperature catalytic carbon monoxide methanation performance via vermiculite-derived silicon carbide-supported nickel nanoparticles. Sustain. Energy Fuels 2019, 3, 965–974. [Google Scholar] [CrossRef]
- Yao, Y.; Yu, F.; Li, J.; Li, J.; Li, Y.; Wang, Z.; Zhu, M.; Shi, Y.; Dai, B.; Guo, X. Two-dimensional NiAl layered double oxides as non-noble metal catalysts for enhanced CO methanation performance at low temperature. Fuel 2019, 255, 115770. [Google Scholar] [CrossRef]
- Zhang, M.; Li, P.; Tian, Z.; Zhu, M.; Wang, F.; Li, J.; Dai, B.; Yu, F.; Qiu, H.; Gao, H. Clarification of active sites at interfaces between silica support and nickel active components for carbon monoxide methanation. Catalysts 2018, 8, 293. [Google Scholar] [CrossRef]
- Mills, G.A.; Steffgen, F.W. Catalytic methanation. Catal. Rev. 1974, 8, 159–210. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Kondarides, D.I.; Verykios, X.E. Selective methanation of CO over supported noble metal catalysts: effects of the nature of the metallic phase on catalytic performance. Appl. Catal. A Gen. 2008, 344, 45–54. [Google Scholar] [CrossRef]
- Derekaya, F.B.; Yaşar, G. The CO methanation over NaY-zeolite supported Ni/Co3O4, Ni/ZrO2, Co3O4/ZrO2 and Ni/Co3O4/ZrO2 catalysts. Catal. Commun. 2011, 13, 73–77. [Google Scholar] [CrossRef]
- Kok, E.; Scott, J.; Cant, N.; Trimm, D. The impact of ruthenium, lanthanum and activation conditions on the methanation activity of alumina-supported cobalt catalysts. Catal. Today 2011, 164, 297–301. [Google Scholar] [CrossRef]
- Habazaki, H.; Yamasaki, M.; Zhang, B.P.; Kawashima, A.; Kohno, S.; Takai, T.; Hashimoto, K. Co-methanation of carbon monoxide and carbon dioxide on supported nickel and cobalt catalysts prepared from amorphous alloys. Appl. Catal. A Gen. 1998, 172, 131–140. [Google Scholar] [CrossRef]
- Nizio, M.; Albarazi, A.; Cavadias, S.; Amouroux, J.; Galvez, M.E.; Da Costa, P. Hybrid plasma-catalytic methanation of CO2 at low temperature over ceria zirconia supported Ni catalysts. Int. J. Hydrog. Energy 2016, 41, 11584–11592. [Google Scholar] [CrossRef]
- Jwa, E.; Mok, Y.S.; Lee, S.B. Nonthermal plasma-assisted catalytic methanation of CO and CO2 over nickel-loaded alumina. In Proceedings of the Energy and Sustainability III 2011, Alicante, Spain, 11–13 April 2011; Volume 143, pp. 361–368. [Google Scholar]
- Nizio, M.; Benrabbah, R.; Krzak, M.; Debek, R.; Motak, M.; Cavadias, S.; Gálvez, M.E.; Da Costa, P. Low temperature hybrid plasma-catalytic methanation over Ni-Ce-Zr hydrotalcite-derived catalysts. Catal. Commun. 2016, 83, 14–17. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Tu, X. Plasma-catalytic CO2 hydrogenation at low temperatures. IEEE Trans. Plasma Sci. 2015, 44, 405–411. [Google Scholar] [CrossRef]
- Lee, C.J.; Lee, D.H.; Kim, T. Enhancement of methanation of carbon dioxide using dielectric barrier discharge on a ruthenium catalyst at atmospheric conditions. Catal. Today 2017, 293, 97–104. [Google Scholar] [CrossRef]
- Cheng, T.; Fang, Z.; Hu, Q.; Han, K.; Yang, X.; Zhang, Y. Low-temperature CO oxidation over CuO/Fe2O3 catalysts. Catal. Commun. 2007, 8, 1167–1171. [Google Scholar] [CrossRef]
- Chen, M.; Goodman, D. The structure of catalytically active gold on titania. Science 2004, 306, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.J.; Liu, Y.; Bongard, H.; Schüth, F. Very low temperature CO oxidation over colloidally deposited gold nanoparticles on Mg(OH)2 and MgO. J. Am. Chem. Soc. 2010, 132, 1520–1522. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, A.; Wang, X.; Zhang, T. Preferential oxidation of CO under excess H2 conditions over iridium catalysts. Int. J. Hydrog. Energy 2007, 32, 3880–3886. [Google Scholar] [CrossRef]
- Haruta, M.; Yamada, N.; Kobayashi, T.; Iijima, S. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Mahammadunnisa, S.; Reddy, P.M.K.; Reddy, E.L.; Subrahmanyam, C. Catalytic DBD plasma reactor for CO oxidation by in situ N2O decomposition. Catal. Today 2013, 211, 53–57. [Google Scholar] [CrossRef]
- Pietruszka, B.; Anklam, K.; Heintze, M. Plasma-assisted partial oxidation of methane to synthesis gas in a dielectric barrier discharge. Appl. Catal. A Gen. 2004, 261, 19–24. [Google Scholar] [CrossRef]
- Zhou, A.; Chen, D.; Dai, B.; Ma, C.; Li, P.; Yu, F. Direct decomposition of CO2 using self-cooling dielectric barrier discharge plasma. Greenh. Gases Sci. Technol. 2017, 7, 721–730. [Google Scholar] [CrossRef]
- Li, R.; Tang, Q.; Yin, S.; Sato, T. Plasma catalysis for CO2 decomposition by using different dielectric materials. Fuel Process. Technol. 2006, 87, 617–622. [Google Scholar] [CrossRef]
- Indarto, A.; Yang, D.R.; Choi, J.W.; Lee, H.; Song, H.K. Gliding arc plasma processing of CO2 conversion. J. Hazard. Mater. 2007, 146, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Gallon, H.J.; Twigg, M.V.; Gorry, P.A.; Whitehead, J.C. Dry reforming of methane over a Ni/Al2O3 catalyst in a coaxial dielectric barrier discharge reactor. J. Phys. D Appl. Phys. 2011, 44, 274007. [Google Scholar] [CrossRef]
- Paulussen, S.; Verheyde, B.; Tu, X.; De Bie, C.; Martens, T.; Petrovic, D.; Bogaerts, A.; Sels, B. Conversion of carbon dioxide to value-added chemicals in atmospheric pressure dielectric barrier discharges. Plasma Sources Sci. Technol. 2010, 19, 034015. [Google Scholar] [CrossRef]
- Ray, D.; Subrahmanyam, C. CO2 decomposition in a packed DBD plasma reactor: influence of packing materials. RSC Adv. 2016, 6, 39492–39499. [Google Scholar] [CrossRef]
- Uytdenhouwen, Y.; Van Alphen, S.; Michielsen, I.; Meynen, V.; Cool, P.; Bogaerts, A. A packed-bed DBD micro plasma reactor for CO2 dissociation: does size matter? Chem. Eng. J. 2018, 348, 557–568. [Google Scholar] [CrossRef]
- Zhou, A.; Chen, D.; Ma, C.; Yu, F.; Dai, B. DBD plasma-ZrO2 catalytic decomposition of CO2 at low temperatures. Catalysts 2018, 8, 256. [Google Scholar] [CrossRef]
- Duan, X.; Hu, Z.; Li, Y.; Wang, B. Effect of dielectric packing materials on the decomposition of carbon dioxide using DBD microplasma reactor. AIChE J. 2015, 61, 898–903. [Google Scholar] [CrossRef]
- Mei, D.; Zhu, X.; Wu, C.; Ashford, B.; Williams, P.T.; Tu, X. Plasma-photocatalytic conversion of CO2 at low temperatures: understanding the synergistic effect of plasma-catalysis. Appl. Catal. B: Environ. 2016, 182, 525–532. [Google Scholar] [CrossRef]
- Shengjie, Z.; Amin, Z.; Feng, Y.; Bin, D.; Cunhua, M. Enhanced CO2 decomposition via metallic foamed electrode packed in self-cooling DBD plasma device. Plasma Sci. Technol. 2019, 21, 085504. [Google Scholar]
- Eliasson, B.; Liu, C.J.; Kogelschatz, U. Direct conversion of methane and carbon dioxide to higher hydrocarbons using catalytic dielectric-barrier discharges with zeolites. Ind. Eng. Chem. Res. 2000, 39, 1221–1227. [Google Scholar] [CrossRef]
- Kraus, M.; Eliasson, B.; Kogelschatz, U.; Wokaun, A. CO2 reforming of methane by the combination of dielectric-barrier discharges and catalysis. Phys. Chem. Chem. Phys. 2001, 3, 294–300. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, H.; Yan, B.; Jin, Y.; Cheng, Y. Steam enhanced carbon dioxide reforming of methane in DBD plasma reactor. Int. J. Hydrog. Energy 2011, 36, 8301–8306. [Google Scholar] [CrossRef]
- Nozaki, T.; Muto, N.; Kado, S.; Okazaki, K. Dissociation of vibrationally excited methane on Ni catalyst: Part 1. application to methane steam reforming. Catal. Today 2004, 89, 57–65. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.H.; Song, Y.H.; Choi, W.C.; Park, Y.K.; Kim, D.H. Synergistic effect of non-thermal plasma–catalysis hybrid system on methane complete oxidation over Pd-based catalysts. Chem. Eng. J. 2015, 259, 761–770. [Google Scholar] [CrossRef]
- Nozaki, T.; Muto, N.; Kado, S.; Okazaki, K. Minimum energy requirement for methane steam reforming in plasma-catalyst reactor. Am. Chem. Soc. Div. Fuel Chem. 2004, 49, 179. [Google Scholar]
- Zhao, D.; Yu, F.; Zhou, A.M.; Ma, C.H.; Dai, B. High-efficiency removal of NOx using dielectric barrier discharge nonthermal plasma with water as an outer electrode. Plasma Sci. Technol. 2018, 20, 14020. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, F.; Zhu, M.; Dan, J.; Wang, X.; Zhang, J.; Dai, B. Enhanced low temperature NO reduction performance via MnOx-Fe2O3/vermiculite monolithic honeycomb catalysts. Catalysts 2018, 8, 100. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, K.; Wang, W.; Wang, F.; Dan, J.; Yang, S.; Zhang, J.; Dai, B.; Yu, F. Enhanced selective catalytic reduction of NO with NH3 via porous micro-spherical aggregates of Mn-Ce-Fe-Ti mixed oxide nanoparticles. Green Energy Environ. 2019, 4, 311–321. [Google Scholar] [CrossRef]
- Tian, J.; Wang, C.; Yu, F.; Zhou, X.; Zhang, J.; Yang, S.; Dan, J.; Cao, P.; Dai, B.; Wang, Q. Mn-Ce-Fe-Al mixed oxide nanoparticles via a high shear mixer facilitated coprecipitation method for low temperature selective catalytic reduction of NO with NH3. Appl. Catal. A Gen. 2019, 4, 117237. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, F.; Ma, C.; Dan, J.; Luo, J.; Dai, B. A critical review of recent progress and perspective in practical denitration application. Catalysts 2019, 9, 771. [Google Scholar] [CrossRef]
- Niu, C.; Niu, J.; Wang, S.; Wang, Z.; Dong, S.; Fan, H.; Hong, Y.; Liu, D. Synergistic effect in one-stage dielectric barrier discharge plasma and Ag/Al2O3 catalytic systems on C2H2-SCR of NOx. Catal. Commun. 2019, 123, 49–53. [Google Scholar] [CrossRef]
- Wang, T.; Liu, H.; Sun, B. Dielectric barrier discharge plasma-assisted catalytic reduction of NOx over Mn-Cu catalyst. In Proceedings of the 2016 International Forum on Energy, Environment and Sustainable Development, Shenzhen, China, 16–17 April 2016. [Google Scholar]
- Jinhai, N.; Zhihui, Z.; Dongping, L.; Qi, W. Low-temperature plasma-catalytic reduction of NOx by C2H2 in the presence of excess oxygen. Plasma Sci. Technol. 2008, 10, 466. [Google Scholar] [CrossRef]
- Yi, Y.; Zhou, J.; Gao, T.; Guo, H.; Zhou, J.; Zhang, J. Continuous and scale-up synthesis of high purity H2O2 by safe gas-phase H2/O2 plasma reaction. AIChE J. 2014, 60, 415–419. [Google Scholar] [CrossRef]
- Li, S.; Cao, X.; Liu, L.; Ma, X. Degradation of thiamethoxam in water by the synergy effect between the plasma discharge and the TiO2 photocatalysis. Desalin. Water Treat. 2015, 53, 3018–3025. [Google Scholar] [CrossRef]
- Du, Y.; Nayak, G.; Oinuma, G.; Peng, Z.; Bruggeman, P.J. Effect of water vapor on plasma morphology, OH and H2O2production in He and Ar atmospheric pressure dielectric barrier discharges. J. Phys. D Appl. Phys. 2017, 50, 145201. [Google Scholar] [CrossRef]
- Vasko, C.A.; Liu, D.X.; Veldhuizen, E.M.V.; Iza, F.; Bruggeman, P.J. Hydrogen peroxide production in an atmospheric pressure RF glow discharge: comparison of models and experiments. Plasma Chem. Plasma Process. 2014, 34, 1081–1099. [Google Scholar] [CrossRef]
- Qu, X.; Hu, S.; Li, P.; Li, Z.; Wang, H.; Ma, H.; Li, W. The effect of embedding N vacancies into g-C3N4 on the photocatalytic H2O2 production ability via H2 plasma treatment. Diam. Relat. Mater. 2018, 86, 159–166. [Google Scholar] [CrossRef]
- Àç, G.H. Scale-up synthesis of hydrogen peroxide from H2/O2 with multiple parallel DBD tubes. Plasma Sci. Technol. 2009, 11, 181–186. [Google Scholar]
- Thevenet, F.; Couble, J.; Brandhorst, M.; Dubois, J.; Puzenat, E.; Guillard, C.; Bianchi, D. Synthesis of hydrogen peroxide using dielectric barrier discharge associated with fibrous materials. Plasma Chem. Plasma Process. 2010, 30, 489–502. [Google Scholar] [CrossRef]
- Yi, Y.; Zhou, J.; Guo, H.; Zhao, J.; Su, J.; Wang, L.; Wang, X.; Gong, W. Safe direct synthesis of high purity H2O2 through a H2/O2 plasma reaction. Angew. Chem. Int. Ed. 2013, 52, 8446–8449. [Google Scholar] [CrossRef] [PubMed]
- Gunasooriya, G.K.K.; Van Bavel, A.P.; Kuipers, H.P.; Saeys, M. Key role of surface hydroxyl groups in C–O activation during Fischer–Tropsch synthesis. ACS Catal. 2016, 6, 3660–3664. [Google Scholar] [CrossRef]
- Hong, J.; Chu, W.; Ying, Y.; Chernavskii, P.A.; Khodakov, A. Plasma-assisted design of supported cobalt catalysts for Fischer-Tropsch synthesis. Stud. Surf. Sci. Catal. 2010, 175, 253–257. [Google Scholar]
- Jiang, Y.; Fu, T.; Jing, L.; Li, Z. A zirconium modified Co/SiO2 Fischer-Tropsch catalyst prepared by dielectric-barrier discharge plasma. J. Energy Chem. 2013, 22, 506–511. [Google Scholar] [CrossRef]
- Al-Harrasi, W.S.; Zhang, K.; Akay, G. Process intensification in gas-to-liquid reactions: plasma promoted Fischer-Tropsch synthesis for hydrocarbons at low temperatures and ambient pressure. Green Process. Synth. 2013, 2, 479–490. [Google Scholar] [CrossRef]
- Liu, C.J.; Xue, B.; Eliasson, B.; He, F.; Li, Y.; Xu, G.H. Methane conversion to higher hydrocarbons in the presence of carbon dioxide using dielectric-barrier discharge plasmas. Plasma Chem. Plasma Process. 2001, 21, 301–310. [Google Scholar] [CrossRef]
- Saleem, F.; Zhang, K.; Harvey, A. Plasma-assisted decomposition of a biomass gasification tar analogue into lower hydrocarbons in a synthetic product gas using a dielectric barrier discharge reactor. Fuel 2019, 235, 1412–1419. [Google Scholar] [CrossRef]
- Roso, M.; Boaretti, C.; Pelizzo, M.G.; Lauria, A.; Modesti, M.; Lorenzetti, A. Nanostructured photocatalysts based on different oxidized graphenes for VOCs removal. Ind. Eng. Chem. Res. 2017, 56, 9980–9992. [Google Scholar] [CrossRef]
- Maciuca, A.; Batiot-Dupeyrat, C.; Tatibouët, J.M. Synergetic effect by coupling photocatalysis with plasma for low VOCs concentration removal from air. Appl. Catal. B Environ. 2012, 125, 432–438. [Google Scholar] [CrossRef]
- Saoud, W.A.; Assadi, A.A.; Guiza, M.; Bouzaza, A.; Aboussaoud, W.; Ouederni, A.; Soutrel, I.; Wolbert, D.; Rtimi, S. Study of synergetic effect, catalytic poisoning and regeneration using dielectric barrier discharge and photocatalysis in a continuous reactor: abatement of pollutants in air mixture system. Appl. Catal. B Environ. 2017, 213, 53–61. [Google Scholar] [CrossRef]
- Mustafa, M.F.; Fu, X.; Liu, Y.; Abbas, Y.; Wang, H.; Lu, W. Volatile organic compounds (VOCs) removal in non-thermal plasma double dielectric barrier discharge reactor. J. Hazard. Mater. 2018, 347, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Assadi, A.A.; Bouzaza, A.; Vallet, C.; Wolbert, D. Use of DBD plasma, photocatalysis, and combined DBD plasma/photocatalysis in a continuous annular reactor for isovaleraldehyde elimination—synergetic effect and byproducts identification. Chem. Eng. J. 2014, 254, 124–132. [Google Scholar] [CrossRef]
- Zhu, T.; Li, J.; Liang, W.; Jin, Y. Synergistic effect of catalyst for oxidation removal of toluene. J. Hazard. Mater. 2009, 165, 1258–1260. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.; Park, M.J.; Kim, S.B.; Kim, H.J.; Baik, L.J.; Jo, Y.M. Effective dielectric barrier discharge reactor operation for decomposition of volatile organic compounds. J. Clean. Prod. 2018, 198, 1232–1238. [Google Scholar] [CrossRef]
- Choi, J.H.; Han, I.; Baik, H.K.; Lee, M.H.; Han, D.W.; Park, J.C.; Lee, I.S.; Song, K.M.; Lim, Y.S. Analysis of sterilization effect by pulsed dielectric barrier discharge. J. Electrost. 2006, 64, 17–22. [Google Scholar] [CrossRef]
- Kostov, K.; Rocha, V.; Koga-Ito, C.; Matos, B.; Algatti, M.; Honda, R.Y.; Kayama, M.; Mota, R.P. Bacterial sterilization by a dielectric barrier discharge (DBD) in air. Surf. Coat. Technol. 2010, 204, 2954–2959. [Google Scholar] [CrossRef]
- Yang, L.; Chengwu, Y.; Jingjing, L.; Rongjie, Y.; Huijuan, W. Experimental research on the sterilization of Escherichia coli and Bacillus subtilis in drinking water by dielectric barrier discharge. Plasma Sci. Technol. 2016, 18, 173–178. [Google Scholar]
- Eto, H.; Ono, Y.; Ogino, A.; Nagatsu, M. Low-temperature sterilization of wrapped materials using flexible sheet-type dielectric barrier discharge. Appl. Phys. Lett. 2008, 93, 221502. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Miyamae, M.; Nagata, M.; Fukumoto, N. Effects of environmental humidity and temperature on sterilization efficiency of dielectric barrier discharge plasmas in atmospheric pressure air. Jpn. J. Appl. Phys. 2011, 50, 01AH03. [Google Scholar] [CrossRef]
- Mastanaiah, N.; Banerjee, P.; Johnson, J.A.; Roy, S. Examining the role of ozone in surface plasma sterilization using dielectric barrier discharge (DBD) plasma. Plasma Process. Polym. 2013, 10, 1120–1133. [Google Scholar] [CrossRef]
- Hong, Y.C.; Ma, S.H.; Kim, K.; Shin, Y.W. Multihole dielectric barrier discharge with asymmetric electrode arrangement in water and application to sterilization of aqua pathogens. Chem. Eng. J. 2019, 374, 133–143. [Google Scholar] [CrossRef]
- Richardson, S.D. Water analysis: emerging contaminants and current issues. Anal. Chem. 2009, 81, 4645–4677. [Google Scholar] [CrossRef] [PubMed]
- Haixia, W.; Zhi, F.; Yanhua, X. Degradation of aniline wastewater using dielectric barrier discharges at atmospheric pressure. Plasma Sci. Technol. 2015, 17, 228–234. [Google Scholar]
- Mok, Y.S.; Jo, J.O.; Lee, H.J.; Ahn, H.T.; Kim, J.T. Application of dielectric barrier discharge reactor immersed in wastewater to the oxidative degradation of organic contaminant. Plasma Chem. Plasma Process. 2007, 27, 51–64. [Google Scholar] [CrossRef]
- Jin, G.; Pingdao, G.; Li, Y.; Fangchuan, Z. Degradation of dye wastewater by ns-pulse DBD plasma. Plasma Sci. Technol. 2013, 15, 928. [Google Scholar]
- Sun, M.Y.; Jin-Oh, J.; Heon-Ju, L. Dielectric barrier discharge plasma-induced photocatalysis and ozonation for the treatment of wastewater. Plasma Sci. Technol. 2008, 10, 100–105. [Google Scholar] [CrossRef]
- Attri, P.; Tochikubo, F.; Park, J.H.; Choi, E.H.; Koga, K.; Shiratani, M. Impact of Gamma rays and DBD plasma treatments on wastewater treatment. Sci. Rep. 2018, 8, 2926. [Google Scholar] [CrossRef]
- Tichonovas, M.; Krugly, E.; Racys, V.; Hippler, R.; Kauneliene, V.; Stasiulaitiene, I.; Martuzevicius, D. Degradation of various textile dyes as wastewater pollutants under dielectric barrier discharge plasma treatment. Chem. Eng. J. 2013, 229, 9–19. [Google Scholar] [CrossRef]
- Kuraica, M.; Obradović, B.; Manojlović, D.; Ostojić, D.; Purić, J. Application of coaxial dielectric barrier discharge for potable and waste water treatment. Ind. Eng. Chem. Res 2006, 45, 882–905. [Google Scholar]
- Tang, S.; Yuan, D.; Rao, Y.; Li, N.; Qi, J.; Cheng, T.; Sun, Z.; Gu, J.; Huang, H. Persulfate activation in gas phase surface discharge plasma for synergetic removal of antibiotic in water. Chem. Eng. J. 2018, 337, 446–454. [Google Scholar] [CrossRef]
- Sarangapani, C.; Misra, N.; Milosavljevic, V.; Bourke, P.; O’Regan, F.; Cullen, P. Pesticide degradation in water using atmospheric air cold plasma. J. Water Process Eng. 2016, 9, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Xingmin, S.; Jinren, L.; Guimin, X.; Yueming, W.; Lingge, G.; Xiaoyan, L.; Yang, Y.; Zhang, G. Effect of low-temperature plasma on the degradation of omethoate residue and quality of apple and spinach. Plasma Sci. Technol. 2018, 20, 044004. [Google Scholar] [Green Version]
- Hu, Y.; Bai, Y.; Li, X.; Chen, J. Application of dielectric barrier discharge plasma for degradation and pathways of dimethoate in aqueous solution. Sep. Purif. Technol. 2013, 120, 191–197. [Google Scholar] [CrossRef]
- Li, S.; Ma, X.; Jiang, Y.; Cao, X. Acetamiprid removal in wastewater by the low-temperature plasma using dielectric barrier discharge. Ecotoxicol. Environ. Saf. 2014, 106, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zheng, Z.; Sun, Y.; Luan, J.; Wang, Z.; Wang, L.; Feng, J. Degradation of diuron in aqueous solution by dielectric barrier discharge. J. Hazard. Mater. 2008, 154, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Jović, M.S.; Dojčinović, B.P.; Kovačević, V.V.; Obradović, B.M.; Kuraica, M.M.; Gašić, U.M.; Roglić, G.M. Effect of different catalysts on mesotrione degradation in water falling film DBD reactor. Chem. Eng. J. 2014, 248, 63–70. [Google Scholar] [CrossRef]
- Wardenier, N.; Vanraes, P.; Nikiforov, A.; Van Hulle, S.W.; Leys, C. Removal of micropollutants from water in a continuous-flow electrical discharge reactor. J. Hazard. Mater. 2019, 362, 238–245. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Molina, R.; Schikora, H.; Müller, M.; Bayona, J.M. Removal of priority pollutants from water by means of dielectric barrier discharge atmospheric plasma. J. Hazard. Mater. 2013, 262, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.; Pankaj, S.; Walsh, T.; O’Regan, F.; Bourke, P.; Cullen, P. In-package nonthermal plasma degradation of pesticides on fresh produce. J. Hazard. Mater. 2014, 271, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]















| Types of Catalyst | Types of Plasma | Characteristics | Remarks | Applications | References |
|---|---|---|---|---|---|
| Pt and Co catalyst | DBD plasma H2/N2/CH4 | Higher selectivity | Methane conversion | [16] | |
| Pt/TiO2 catalyst | DBD cold plasma Ar & H2 | Smaller Pt particles homogeneously distributed | Size of particles: 1.7 nm | Mesoporous photocatalyst with enhanced activity | [17] |
| Pd/Graphene sheets | DBD plasma-assisted H2 | Well-dispersed and higher catalytic efficiency | Size of particles: 2 nm | The hydrodesulfu-rization of carbonyl sulfide | [25] |
| Au/TiO2 | DBD plasma O2 | Higher activity small particle size and narrow size distribution | Size of particles: 2.6 nm | CO oxidation | [26] |
| Au/P25-PC | AP DBD cold plasma Ar & H2 | Smaller size and higher CO oxidation activity | Size of particles: 4.6 nm | CO oxidation | [27] |
| Ni/MgO | DBD plasma H2 | Smaller particle size and higher specific surface area | Size of particles: 3.4 nm | CO2 reforming of methane | [28] |
| Ni/Ce/SBA-15-P | DBD plasma Ar & H2 | High specific surface area and good high-temperature stability | Size of particles: 7.1 nm | Methanation of carbon oxides | [29] |
| Ni/SiO2 | Ammonia impregnation combined with DBD NH3 | High dispersion and high temperature stability | Size of particles: 11.2 nm | CO methanation | [30] |
| Ni/ZrO2 | DBD plasma H2 | Highly dispersed and long-time stability | Size of particles: 10.6 nm | CO methanation | [31] |
| Ni/MgO | DBD plasma air | Low temperature activity and good stability | Size of particles: 5.4 nm | CO2 reforming of methane | [32] |
| Ni/MgAl2O4 | DBD plasma air | High dispersion and unique structure | Size of particles: 8.9 nm | CO2 methanation | [33] |
| Ni/PVMT | DBD plasma H2 | Ultralow Ni loading and excellent catalytic performance | CO methanation | [34] | |
| Ni/SiO2 | DBD plasma air | Low activity with small size and coke resistance | Size of particles: 7.1 nm | Steam reforming of methane | [35] |
| MnO | Wire-plate DBD O2 | Low cost, environmentally friendly and high activity | Toluene decomposition | [36] | |
| Co/CNTs | DBD plasma Ar | Uniform and dispersed Co particles | FTs | [37] | |
| W2N | DBD plasma NH3 | Excellent chemical stability and high performance | Size of particles: 5.99 nm | Acetylene hydrochlorina-tion | [38] |
| TiO2-β zeolite | DBD plasma O2 | Higher methanol photodegradation rates | The BET specific surface area: 421.9 m2/g | Methanol photooxidatin | [39] |
| Types of Catalyst | Types of Plasma | Characteristics | Remarks | Applications | References |
|---|---|---|---|---|---|
| Multi-wall CNTs | DBD plasma O2 | Generate oxygen-containing group | The BET specific surface area: 156 m2/g | Surface modification | [50] |
| Graphite | DBD plasma air | Create novel defects | Protrusions 1−5 nm in diameter and smooth depressions 5−7 nm wide | Modification of graphite surfaces | [51] |
| Co3O4 nanosheets | Plasma-engraved Ar | With high surface area and oxygen vacancies | The BET specific surface area: 160.26 m2/g | OER | [52] |
| Ag catalysts | Plasma-activated H2, Ar & O2 | Lower overpotential | Pore-like defects 50−100 nm in size | Carbon dioxide electroreduction | [53] |
| Ar-TiO2 | DBD plasma Ar | Oxygen vacancies and Ti3+ defects | The energy band gap reduction | Photocatalytic degradation of organic dyes | [54] |
| Teflon films | DBD H2O | Point electrode | Prevent degradation and erosion | [55] |
| Types of Catalyst | Types of Plasma | Characteristics | Remarks | Applications | References |
|---|---|---|---|---|---|
| N-doped ZnO thin films | DBD pulsed laser deposition N2 | Low temperature photoluminescence spectra | The maximum hole density: 10−17–10−18·cm−3 | Make ZnO based electronic devices | [56] |
| N-PEGO | DBD plasma CO2 & NH3 | High onset potential and good electrocatalysis stability | The BET specific surface area: 380.0 m2/g | ORR | [57] |
| N-vacancy-doped g-C3N4 | DBD plasma H2 | Outstanding photocatalytic | H2O2 production | [58] | |
| Cu2O/N-TiO2 | DBD plasma N2/Ar | Dispersed uniformly and higher photodegradation efficiency | Improved photocatalytic activity | [59] | |
| S-doped g-C3N4 | DBD plasma H2S | Larger specific surface area | The BET specific surface area: 52.8 m2/g | Enhanced photocatalytic performance | [60] |
| P-Co3O4 | Ar plasma | Porous, discontinuous, and loose surface | HER and OER | [61] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Ma, C.; Zhu, S.; Yu, F.; Dai, B.; Yang, D. A Review of Recent Advances of Dielectric Barrier Discharge Plasma in Catalysis. Nanomaterials 2019, 9, 1428. https://doi.org/10.3390/nano9101428
Li J, Ma C, Zhu S, Yu F, Dai B, Yang D. A Review of Recent Advances of Dielectric Barrier Discharge Plasma in Catalysis. Nanomaterials. 2019; 9(10):1428. https://doi.org/10.3390/nano9101428
Chicago/Turabian StyleLi, Ju, Cunhua Ma, Shengjie Zhu, Feng Yu, Bin Dai, and Dezheng Yang. 2019. "A Review of Recent Advances of Dielectric Barrier Discharge Plasma in Catalysis" Nanomaterials 9, no. 10: 1428. https://doi.org/10.3390/nano9101428
APA StyleLi, J., Ma, C., Zhu, S., Yu, F., Dai, B., & Yang, D. (2019). A Review of Recent Advances of Dielectric Barrier Discharge Plasma in Catalysis. Nanomaterials, 9(10), 1428. https://doi.org/10.3390/nano9101428