Silver Quantum Dot Decorated 2D-SnO2 Nanoflakes for Photocatalytic Degradation of the Water Pollutant Rhodamine B
Abstract
:1. Introduction
2. Materials and Methods
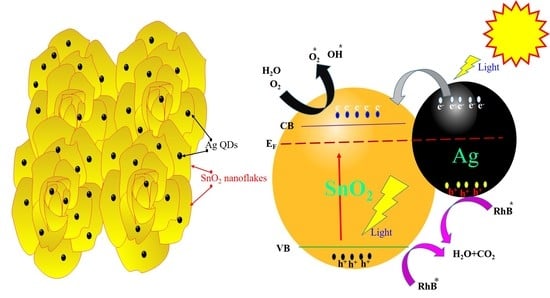
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Zhuang, J.; Wang, X. SnO2 quantum dots and quantum wires: Controllable synthesis, self-assembled 2D architectures, and gas-sensing properties. J. Am. Chem. Soc. 2008, 130, 12527–12535. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luan, D.; Boey, F.Y.; Lou, X.W. Formation of SnO2 hollow nanospheres inside mesoporous silica nanoreactors. J. Am. Chem. Soc. 2011, 133, 4738–4741. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, Y.; Ge, M.; Xu, X.; Zhang, Z.; Jiang, J.Z. Large-scale synthesis of SnO2 nanosheets with high lithium storage capacity. J. Am. Chem. Soc. 2009, 132, 46–47. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Jin, M.; Xie, S.; Kuang, Q.; Jiang, Z.; Jiang, Y.; Xie, Z.; Zheng, L. Synthesis of tin dioxide octahedral nanoparticles with exposed high-energy {221} facets and enhanced gas-sensing properties. Angew. Chem. Int. Ed. 2009, 48, 9180–9183. [Google Scholar] [CrossRef]
- Pan, Z.W.; Dai, Z.R.; Wang, Z.L. Nanobelts of semiconducting oxides. Science 2001, 291, 1947–1949. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef]
- Wang, H.; Rogach, A.L. Hierarchical SnO2 nanostructures: Recent advances in design, synthesis, and applications. Chem. Mater. 2014, 26, 123–133. [Google Scholar] [CrossRef]
- Maiti, A.; Rodriguez, J.A.; Law, M.; Kung, P.; McKinney, J.R.; Yang, P. SnO2 nanoribbons as NO2 sensors: Insights from first principles calculations. Nano Lett. 2003, 3, 1025–1028. [Google Scholar] [CrossRef]
- Qian, J.F.; Liu, P.; Xiao, Y.; Jiang, Y.; Cao, Y.L.; Ai, X.P.; Yang, H.X. TiO2-coated multilayered SnO2 hollow microspheres for dye-sensitized solar cells. Adv. Mater. 2009, 21, 3663–3667. [Google Scholar] [CrossRef]
- Ding, S.; Luan, D.; Boey, F.Y.C.; Chen, J.S.; Lou, X.W. SnO2 nanosheets grown on graphene sheets with enhanced lithium storage properties. Chem. Commun. 2011, 47, 7155–7157. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Lee, J.Y. Hollow Core–shell mesospheres of crystalline SnO2 nanoparticle aggregates for high capacity Li+ ion storage. Chem. Mater. 2008, 20, 1841–1846. [Google Scholar] [CrossRef]
- Dutta, S.K.; Mehetor, S.K.; Pradhan, N. Metal semiconductor heterostructures for photocatalytic conversion of light energy. J. Phys. Chem. Lett. 2015, 6, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhou, Y.; Cao, J.; Sheng, Y.; Wu, Y.; Cui, C.; Li, C.; Feng, B. Fabrication of carbon quantum dots modified granular SnO2 nanotubes for visible light Photocatalysis. Mater. Lett. 2016, 170, 187–191. [Google Scholar] [CrossRef]
- Pincella, F.; Isozaki, K.; Miki, K. A visible light-driven plasmonic photocatalyst. Light Sci. Appl. 2014, 3, e133. [Google Scholar] [CrossRef]
- Zhang, D.F.; Sun, L.D.; Yin, J.L.; Yan, C.H. Low-temperature fabrication of highly crystalline SnO2 nanorods. Adv. Mater. 2003, 15, 1022–1025. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, X.; Xia, Y. A solution-phase, precursor route to polycrystalline SnO2 nanowires that can be used for gas sensing under ambient conditions. J. Am. Chem. Soc. 2003, 125, 16176–16177. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, H.C.; Lee, J.Y. Synthesis of new-phased VooH hollow “dandelions” and their application in lithium-ion batteries. Adv. Mater. 2006, 18, 645–649. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Y.; Zuo, Y.; Li, C.; Yang, J.; Lu, C. Synthesis and photocatalysis of hierarchical heteroassemblies of ZnO branched nanorod arrays on Ag core nanowires. Nanoscale 2012, 4, 5895–5901. [Google Scholar] [CrossRef]
- Wang, S.; Yun, J.H.; Luo, B.; Butburee, T.; Peerakiatkhajohn, P.; Thaweesak, S.; Wang, S. Recent Progress on Visible Light Responsive Heterojunctions for Photocatalytic Applications. J. Mater. Sci. Technol. 2017, 33, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.W.; Wi, D.H.; Lee, S.; Han, S.W. Metal-semiconductor heteronanocrystals with desired configurations for plasmonic photocatalysis. J. Am. Chem. Soc. 2016, 138, 15766–15773. [Google Scholar] [CrossRef] [PubMed]
- Shahar, Y.B.; Banin, U. Hybrid semiconductor-metal nanorods as photocatalysts. Top. Curr. Chem. 2016, 374, 54. [Google Scholar] [CrossRef] [PubMed]
- Babu, B.; Cho, M.; Byon, C.; Shim, J. One pot synthesis of Ag-SnO2 quantum dots for highly enhanced sunlight-driven photocatalytic activity. J. Alloys Compd. 2018, 731, 162–171. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, G.; Liu, Y.; Zhou, X.; Zhu, Y.; Shi, D.; Zhang, P.; Cao, X.; Wang, B. The role of Sn in enhancing the visible-light photocatalytic activity of hollow hierarchical microspheres of the Bi/BioBr heterojunction. Phys. Chem. Chem. Phys. 2015, 17, 8078–8086. [Google Scholar] [CrossRef]
- Zhao, Q.R.; Gao, Y.; Bai, X.; Wu, C.Z.; Xie, Y. Facile synthesis of SnO2 hollow nanospheres and applications in gas sensors and electrocatalysts. Eur. J. Inorg. Chem. 2006, 8, 1643–1648. [Google Scholar] [CrossRef]
- Ansari, S.; Khan, M.; Ansari, M.; Lee, J.; Cho, M. Visible light-driven photocatalytic and photoelectrochemical studies of AgSnO2 nanocomposites synthesized using an electrochemically active biofilm. RSC Adv. 2014, 4, 26013–26021. [Google Scholar] [CrossRef]
- Ramgir, N.S.; Mulla, I.S.; Vijayamohanan, K.P. Effect of RuO2 in the shape selectivity of submicron-sized SnO2 structures. J. Phys. Chem. B. 2005, 109, 12297–12303. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Lee, J.; Cho, M.H. Highly photoactive SnO2 nanostructures engineered by electrochemically active biofilm. New J. Chem. 2014, 38, 2462–2469. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, Z.; Luo, C.; Qiao, X. One-pot green synthesis of Ag-decorated SnO2 microsphere: An efficient and reusable catalyst for reduction of 4-Nitrophenol. Nanoscale Res. Lett. 2017, 12, 435. [Google Scholar] [CrossRef]
- Kaur, H.; Bhatti, H.S.; Singh, K. Dopant incorporation in ultra-small quantum dots: A case study on the effect of dopant concentration on lattice and properties of SnO2 QDs. J. Mater. Sci. Mater. Electron. 2019, 30, 2246–2264. [Google Scholar] [CrossRef]
- Mallikarjuna, K.; Kotesh Kumar, M.; Subba Reddy, B.V.; Kim, H. Hydrogen Production from Water Splitting: Fabrication of ZnO Nanorod Decorated Cu NW Heterogeneous Hybrid Structures for Photocatalytic Applications. J. Clust. Sci. 2019, 30, 449–457. [Google Scholar] [CrossRef]
- Prakasha, K.; Senthil Kumar, P.; Latha, P.; Stalin Durai, K.; Shanmugam, R.; Karuthapandian, S. Dry synthesis of water lily flower like SrO2/g-C3N4 nanohybrids for the visible light induced superior photocatalytic activity. Mater. Res. Bull. 2017, 93, 112–122. [Google Scholar]
- Bathula, B.; Koutavarapu, R.; Harish, V.V.N.; Shim, J.; Yoo, K. Novel in-situ synthesis of Au/SnO2 quantum dots for enhanced visible-light driven photocatalytic applications. Ceram. Int. 2019, 45, 5743–5750. [Google Scholar]
- Sun, L.; Wu, W.; Yang, S.; Zhou, J.; Hong, M.; Xiao, X.; Ren, F.; Jiang, C. Template and silica interlayer tailorable synthesis of spindle-like multilayer α-Fe2O3/Ag/SnO2 ternary hybrid architectures and their enhanced photocatalytic activity. ACS Appl. Mater. Interfaces 2014, 6, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.; Chen, Z.; Qin, L.; Yang, L.; Zhu, L.; Tang, P.; Gao, T.; Huang, Y.; Sha, Z.; et al. Visible light induced photocatalysis on CdS quantum dots decorated TiO2 nanotube arrays. Appl. Catal. A Gen. 2015, 498, 159–166. [Google Scholar] [CrossRef]
- Akhundi, A.; Yangieh, A.H. A simple large-scale method for preparation of g-C3N4/SnO2 nanocomposite as visible–light-driven photocatalyst for degradation of an organic pollutant. Mater. Exp. 2015, 5, 309–318. [Google Scholar] [CrossRef]
- Yu, H.-B.; Rong, Z.J.; Lu, Y.; Wang, X.H.; Luo, X.B. Preparation of CeO2 quantum dots/Cu2O nanocomposites with enhanced photocatalytic properties. J. Nanosci. Nanotechnol. 2018, 18, 5794–5798. [Google Scholar] [CrossRef]
- Liu, X.; Chen, K.; Shim, J.J.; Huang, J. Facile synthesis of porous Fe2O3 nanorods and their photocatalytic properties. J. Saudi Chem. Soc. 2015, 19, 479–484. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Q.; Wang, Z.; Wang, G.; Zhang, B.; Zhang, Q.; Yang, D. Rapid fabrication of SnO2 nanoparticle photocatalyst: Computational understanding and photocatalytic degradation of organic dye. Inorg. Chem. Front. 2018, 5, 3005–3014. [Google Scholar] [CrossRef]
- Albiter, E.; Valenzuela, M.A.; Alfaro, S.; Valverde-Aguilar, G.; Martínez-Pallares, F.M. Photocatalytic deposition of Ag nanoparticles on TiO2: Metal precursor effect on the structural and photoactivity properties. J. Saudi Chem. Soc. 2015, 19, 563–573. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Y.; Zhang, Z.; Liu, X.; Jia, H.; Xu, B. Synthesis of spherical Ag/ZnO heterostructural composites with excellent photocatalytic activity under visible light and UV irradiation. Appl. Surf. Sci. 2015, 355, 644–652. [Google Scholar] [CrossRef]
- Yu, K.; Yang, S.; He, H.; Sun, C.; Gu, C.; Ju, Y. Visible Light-Driven Photocatalytic Degradation of Rhodamine B over NaBiO3: Pathways and Mechanism. J. Phys. Chem. A 2009, 113, 10024–10032. [Google Scholar] [CrossRef] [PubMed]










| S. NO | Photocatalyst | Synthesis Method | Dye | Light Source | Degradation Time (min) | Ref. |
|---|---|---|---|---|---|---|
| 1 | Ag/SnO2 QDs | One-pot | RhB | Sunlight | 180 | [23] |
| 2 | SrO2/g-C3N4 | Dry | RhB | Visible | 60 | [32] |
| 3 | Au/ SnO2 QDs | Solvothermal | RhB | Visible | 200 | [33] |
| 4 | α-Fe2O3/Ag/SiO2/SnO2 | Template | RhB | UV & Visible | 180 | [34] |
| 5 | CdS QDs/TiO2 | Electrochemical | RhB | Visible | 300 | [35] |
| 6 | g-C3N4/ SnO2 | Refluxing | RhB | Visible | 420 | [36] |
| 7 | CeO2-QDs/Cu2O | Hydrothermal | RhB | Simulated sunlight | 180 | [37] |
| 8 | Fe2O3 Nanorods | Chemical | RhB | Simulated solar | 270 | [38] |
| 9 | SnO2 | Chemical | RhB | UV light | 270 | [39] |
| 10 | Ag-TiO2-P25 | Sonochemical | RhB | Simulated solar | 100 | [40] |
| 11 | Ag/ZnO | Chemical | RhB | Visible & UV | 90 | [41] |
| 12 | Ag QDs/SnO2 | Hydrothermal | RhB | Simulated solar | 40 | (Present Study) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siva Kumar, N.; Asif, M.; Ranjeth Kumar Reddy, T.; Shanmugam, G.; Ajbar, A. Silver Quantum Dot Decorated 2D-SnO2 Nanoflakes for Photocatalytic Degradation of the Water Pollutant Rhodamine B. Nanomaterials 2019, 9, 1536. https://doi.org/10.3390/nano9111536
Siva Kumar N, Asif M, Ranjeth Kumar Reddy T, Shanmugam G, Ajbar A. Silver Quantum Dot Decorated 2D-SnO2 Nanoflakes for Photocatalytic Degradation of the Water Pollutant Rhodamine B. Nanomaterials. 2019; 9(11):1536. https://doi.org/10.3390/nano9111536
Chicago/Turabian StyleSiva Kumar, Nadavala, Mohammad Asif, T. Ranjeth Kumar Reddy, Gnanendra Shanmugam, and Abdelhamid Ajbar. 2019. "Silver Quantum Dot Decorated 2D-SnO2 Nanoflakes for Photocatalytic Degradation of the Water Pollutant Rhodamine B" Nanomaterials 9, no. 11: 1536. https://doi.org/10.3390/nano9111536
APA StyleSiva Kumar, N., Asif, M., Ranjeth Kumar Reddy, T., Shanmugam, G., & Ajbar, A. (2019). Silver Quantum Dot Decorated 2D-SnO2 Nanoflakes for Photocatalytic Degradation of the Water Pollutant Rhodamine B. Nanomaterials, 9(11), 1536. https://doi.org/10.3390/nano9111536