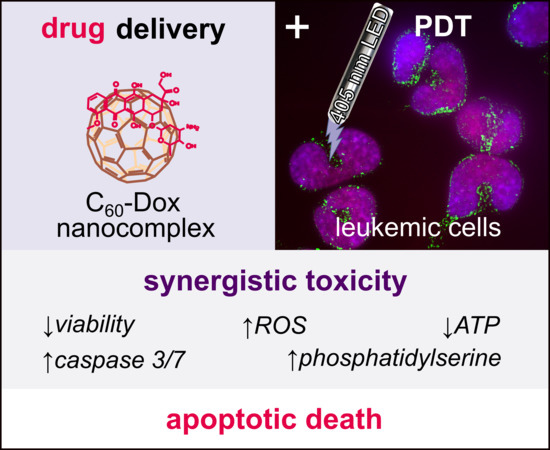
Synergy of Chemo- and Photodynamic Therapies with C60 Fullerene-Doxorubicin Nanocomplex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. C60 and C60-Dox Nanocomplex
2.3. LED Light Source for Photodynamic Therapy
2.4. Cell Culture
2.5. Immunofluorescence Staining of C60
2.6. Fluorescence Microscopy
2.7. Photodynamic Therapy In Vitro and Cell Viability Assay
2.8. Intracellular Reacrive Oxygen Species Generation
2.9. Intercellular ATP Content
2.10. Caspase 3/7 Activity
2.11. Flow Cytometry Analysis
2.12. Statistics
3. Results
3.1. Localization of C60 and Dox in Cells Treated with C60-Dox Nanocomplexes
3.2. Cell Viability
3.3. Intracellular Reacrive Oxygen Species Generation
3.4. Intracellular ATP Content
3.5. Apoptosis Induction
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chabner, B.A.; Roberts, T.G. Timeline: Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Kizek, R.; Adam, V.; Hrabeta, J.; Eckschlager, T.; Smutny, S.; Burda, J.V.; Frei, E.; Stiborova, M. Anthracyclines and ellipticines as DNA-damaging anticancer drugs: Recent advances. Pharmacol. Ther. 2012, 133, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Finn, N.A.; Findley, H.W.; Kemp, M.L. A switching mechanism in doxorubicin bioactivation can be exploited to control doxorubicin toxicity. PLoS Comput. Biol. 2011, 7, e1002151. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Reszka, R. Mitochondria as subcellular targets for clinically useful anthracyclines. Adv. Drug Deliv. Rev. 2001, 49, 87–105. [Google Scholar] [CrossRef]
- Li, L.; Xie, J.; Zhang, X.; Chen, J.; Luo, Y.; Zhang, L.; Luo, R. Retrospective study of photodynamic therapy vs. photodynamic therapy combined with chemotherapy and chemotherapy alone on advanced esophageal cancer. Photodiagnosis Photodyn. Ther. 2010, 7, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem. Rev. 2017, 117, 13566–13638. [Google Scholar] [CrossRef]
- Wang, X.; Meng, G.; Zhang, S.; Liu, X. A Reactive 1O2-Responsive Combined Treatment System of Photodynamic and Chemotherapy for Cancer. Sci. Rep. 2016, 6, 29911. [Google Scholar] [CrossRef]
- Khaliq, N.U.; Park, D.Y.; Lee, H.J.; Oh, K.S.; Seo, J.H.; Kim, S.Y.; Hwang, C.S.; Lim, T.-H.; Yuk, S.H. Pluronic/Heparin Nanoparticles for Chemo-Photodynamic Combination Cancer Therapy through Photoinduced Caspase-3 Activation. ACS Appl. Nano Mater. 2018, 1, 2943–2952. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Cao, Z.; Zhang, X.-J.; Sun, R.; Yu, C.-S.; Yang, X. Cascade-amplifying synergistic effects of chemo-photodynamic therapy using ROS-responsive polymeric nanocarriers. Theranostics 2018, 8, 2939–2953. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.-R.; Chen, S.-F.; Peng, X.-H.; Zheng, Q.-F.; Zheng, B.-Y.; Yeh, C.-K.; Huang, J.-D. A tumor-targeted activatable phthalocyanine-tetrapeptide-doxorubicin conjugate for synergistic chemo-photodynamic therapy. Eur. J. Med. Chem. 2017, 127, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-L.; Lai, P.-S.; Lin, F.-H.; Yueh-Hsiu Wu, S.; Shieh, M.-J. Dual chemotherapy and photodynamic therapy in an HT-29 human colon cancer xenograft model using SN-38-loaded chlorin-core star block copolymer micelles. Biomaterials 2009, 30, 3614–3625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, F.; Ren, C.; Yang, L.; Liu, J.; Cheng, Z.; Chu, L.; Liu, J. Targeted Chemo-Photodynamic Combination Platform Based on the DOX Prodrug Nanoparticles for Enhanced Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 13016–13028. [Google Scholar] [CrossRef] [PubMed]
- Candido, N.M.; de Melo, M.T.; Franchi, L.P.; Primo, F.L.; Tedesco, A.C.; Rahal, P.; Calmon, M.F. Combining Photodynamic Therapy and Chemotherapy: Improving Breast Cancer Treatment with Nanotechnology. J. Biomed. Nanotechnol. 2018, 14, 994–1008. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Ma, Y.-T. Synthesis, characterization, and biological verification of anti-HER2 indocyanine green-doxorubicin-loaded polyethyleneimine-coated perfluorocarbon double nanoemulsions for targeted photochemotherapy of breast cancer cells. J. Nanobiotechnol. 2017, 15, 41. [Google Scholar] [CrossRef]
- Ribeiro, J.N.; da Silva, A.R.; Jorge, R.A. Involvement of mitochondria in apoptosis of cancer cells induced by photodynamic therapy. J. Bras. Patol. Med. Lab. 2004, 40, 383–390. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA A Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Lu, D.Y.; Chen, E.H.; Ding, J.; Xu, B.; Lu, T. R Anticancer drug combinations, a big momentum is needed. J. Postgenom. Drug Biomark. Dev. 2015, 5, e139. [Google Scholar] [CrossRef]
- Aniogo, E.C.; George, B.P.A.; Abrahamse, H. In vitro combined effect of Doxorubicin and sulfonated zinc Phthalocyanine-mediated photodynamic therapy on MCF-7 breast cancer cells. Tumor Biol. 2017, 39, 1010428317727278. [Google Scholar] [CrossRef]
- Wu, C.; He, Q.; Zhu, A.; Li, D.; Xu, M.; Yang, H.; Liu, Y. Synergistic anticancer activity of photo- and chemoresponsive nanoformulation based on polylysine-functionalized graphene. ACS Appl. Mater. Interfaces 2014, 6, 21615–21623. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, X.; Pardhi, D.; Wu, Q.; Zheng, Y.; Zhu, H.; Mao, Z. Folic acid modified cell membrane capsules encapsulating doxorubicin and indocyanine green for highly effective combinational therapy in vivo. Acta Biomater. 2018, 74, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical engineers get to revisit an old friend. Mater. Today 2017, 20, 460–480. [Google Scholar] [CrossRef]
- Sharma, S.K.; Chiang, L.Y.; Hamblin, M.R. Photodynamic therapy with fullerenes in vivo: Reality or a dream? Nanomedicine 2011, 6, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- Scharff, P.; Ritter, U.; Matyshevska, O.P.; Prylutska, S.V.; Grynyuk, I.I.; Golub, A.A.; Prylutskyy, Y.I.; Burlaka, A.P. Therapeutic reactive oxygen generation. Tumori J. 2008, 94, 278–283. [Google Scholar] [CrossRef]
- Tabata, Y.; Murakami, Y.; Ikada, Y. Photodynamic effect of polyethylene glycol-modified fullerene on tumor. Jpn. J. Cancer Res. 1997, 88, 1108–1116. [Google Scholar] [CrossRef]
- Orlova, M. Perspectives of Fullerene Derivatives in PDT and Radiotherapy of Cancers. Br. J. Med. Med Res. 2013, 3, 1731–1756. [Google Scholar] [CrossRef] [Green Version]
- Foley, S.; Crowley, C.; Smaihi, M.; Bonfils, C.; Erlanger, B.F.; Seta, P.; Larroque, C. Cellular localisation of a water-soluble fullerene derivative. Biochem. Biophys. Res. Commun. 2002, 294, 116–119. [Google Scholar] [CrossRef]
- Chirico, F.; Fumelli, C.; Marconi, A.; Tinari, A.; Straface, E.; Malorni, W.; Pellicciari, R.; Pincelli, C. Carboxyfullerenes localize within mitochondria and prevent the UVB-induced intrinsic apoptotic pathway. Exp. Dermatol. 2007, 16, 429–436. [Google Scholar] [CrossRef]
- Grebinyk, A.; Grebinyk, S.; Prylutska, S.; Ritter, U.; Matyshevska, O.; Dandekar, T.; Frohme, M. C60 fullerene accumulation in human leukemic cells and perspectives of LED-mediated photodynamic therapy. Free Radic. Biol. Med. 2018, 124, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Grebinyk, A.; Grebinyk, S.; Prylutska, S.; Ritter, U.; Matyshevska, O.; Dandekar, T.; Frohme, M. HPLC-ESI-MS method for C60 fullerene mitochondrial content quantification. Data Brief 2018, 19, 2047–2052. [Google Scholar] [CrossRef] [PubMed]
- Prylutska, S.V.; Grynyuk, I.I.; Grebinyk, S.M.; Matyshevska, O.P.; Prylutskyy, Y.I.; Ritter, U.; Siegmund, C.; Scharff, P. Comparative study of biological action of fullerenes C60 and carbon nanotubes in thymus cells. Materialwissenschaft und Werkstofftechnik 2009, 40, 238–241. [Google Scholar] [CrossRef]
- Prylutska, S.V.; Matyshevska, O.P.; Golub, A.A.; Prylutskyy, Y.I.; Potebnya, G.P.; Ritter, U.; Scharff, P. Study of C60 fullerenes and C60-containing composites cytotoxicity in vitro. Mater. Sci. Eng. C 2007, 27, 1121–1124. [Google Scholar] [CrossRef]
- Burlaka, A.P.; Sidorik, Y.P.; Prylutska, S.V.; Matyshevska, O.P.; Golub, O.A.; Prylutskyy, Y.I.; Scharff, P. Catalytic system of the reactive oxygen species on the C60 fullerene basis. Exp. Oncol. 2004, 26, 326–327. [Google Scholar] [PubMed]
- Prylutska, S.V.; Grynyuk, I.I.; Palyvoda, K.O.; Matyshevska, O.P. Photoinduced cytotoxic effect of fullerenes C60 on transformed T-lymphocytes. Exp. Oncol. 2010, 32, 29–32. [Google Scholar]
- Grebinyk, S.M.; Palyvoda, K.O.; Prylutska, S.V.; Grynyuk, I.I.; Samoylenko, A.A.; Drobot, L.B.; Matyshevska, O.P. Photoactivated fullerene C60 induces store-operated Ca2+ entry and cytochrome c release in Jurkat cells. Ukr Biokhim Zh (1999) 2012, 84, 58–63. [Google Scholar]
- Mroz, P.; Tegos, G.P.; Gali, H.; Wharton, T.; Sarna, T.; Hamblin, M.R. Photodynamic therapy with fullerenes. Photochem. Photobiol. Sci. 2007, 6, 1139–1149. [Google Scholar] [CrossRef] [Green Version]
- Palyvoda, K.O.; Grynyuk, I.I.; Prylutska, S.V.; Samoylenko, A.A.; Drobot, L.B.; Matyshevska, O.P. Apoptosis photoinduction by C60 fullerene in human leukemic T cells. Ukr Biokhim Zh (1999) 2010, 82, 121–127. [Google Scholar]
- Grynyuk, I.; Grebinyk, S.; Prylutska, S.; Mykhailova, A.; Franskevich, D.; Matyshevska, O.; Schütze, C.; Ritter, U. Photoexcited fullerene C60 disturbs prooxidant-antioxidant balance in leukemic L1210 cells. Materialwissenschaft und Werkstofftechnik 2013, 44, 139–143. [Google Scholar] [CrossRef]
- Montellano, A.; Da Ros, T.; Bianco, A.; Prato, M. Fullerene C60 as a multifunctional system for drug and gene delivery. Nanoscale 2011, 3, 4035–4041. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Raza, K. C60-fullerenes as Drug Delivery Carriers for Anticancer Agents: Promises and Hurdles. Pharm. Nanotechnol. 2017, 5, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Ritter, U.; Prylutskyy, Y.I.; Evstigneev, M.P.; Davidenko, N.A.; Cherepanov, V.V.; Senenko, A.I.; Marchenko, O.A.; Naumovets, A.G. Structural Features of Highly Stable Reproducible C60 Fullerene Aqueous Colloid Solution Probed by Various Techniques. Fuller. Nanotub. Carbon Nanostruct. 2015, 23, 530–534. [Google Scholar] [CrossRef]
- Prylutskyy, Y.I.; Buchelnikov, A.S.; Voronin, D.P.; Kostjukov, V.V.; Ritter, U.; Parkinson, J.A.; Evstigneev, M.P. C60 fullerene aggregation in aqueous solution. Phys. Chem. Chem. Phys. 2013, 15, 9351–9360. [Google Scholar] [CrossRef]
- Prylutskyy, Y.I.; Evstigneev, M.P.; Cherepanov, V.V.; Kyzyma, O.A.; Bulavin, L.A.; Davidenko, N.A.; Scharff, P. Structural organization of C60 fullerene, doxorubicin, and their complex in physiological solution as promising antitumor agents. J. Nanopart. Res. 2015, 17, 45. [Google Scholar] [CrossRef]
- Prylutskyy, Y.I.; Evstigneev, M.P.; Pashkova, I.S.; Wyrzykowski, D.; Woziwodzka, A.; Gołuński, G.; Piosik, J.; Cherepanov, V.V.; Ritter, U. Characterization of C60 fullerene complexation with antibiotic doxorubicin. Phys. Chem. Chem. Phys. 2014, 16, 23164–23172. [Google Scholar] [CrossRef]
- Mosunov, A.; Evstigneev, V.; Buchelnikov, A.; Salo, V.; Prylutskyy, Y.; Evstigneev, M. General up-scaled model of ligand binding with C60 fullerene clusters in aqueous solution. Chem. Phys. Lett. 2019, 721, 22–26. [Google Scholar] [CrossRef]
- Evstigneev, M.P.; Buchelnikov, A.S.; Voronin, D.P.; Rubin, Y.V.; Belous, L.F.; Prylutskyy, Y.I.; Ritter, U. Complexation of C60 fullerene with aromatic drugs. ChemPhysChem 2013, 14, 568–578. [Google Scholar] [CrossRef]
- Panchuk, R.R.; Prylutska, S.V.; Chumakl, V.V.; Skorokhyd, N.R.; Lehka, L.V.; Evstigneev, M.P.; Prylutskyy, Y.I.; Berger, W.; Heffeter, P.; Scharff, P.; et al. Application of C60 Fullerene-Doxorubicin Complex for Tumor Cell Treatment In Vitro and In Vivo. J. Biomed. Nanotechnol. 2015, 11, 1139–1152. [Google Scholar] [CrossRef]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- Chou, T.-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Motlagh, N.S.H.; Parvin, P.; Ghasemi, F.; Atyabi, F. Fluorescence properties of several chemotherapy drugs: Doxorubicin, paclitaxel and bleomycin. Biomed. Opt. Express 2016, 7, 2400–2406. [Google Scholar] [CrossRef] [PubMed]
- Changenet-Barret, P.; Gustavsson, T.; Markovitsi, D.; Manet, I.; Monti, S. Unravelling molecular mechanisms in the fluorescence spectra of doxorubicin in aqueous solution by femtosecond fluorescence spectroscopy. Phys. Chem. Chem. Phys. 2013, 15, 2937–2944. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Hardt, J.I.; Quick, K.L.; Kim-Han, J.S.; Erlanger, B.F.; Huang, T.-T.; Epstein, C.J.; Dugan, L.L. A biologically effective fullerene (C60) derivative with superoxide dismutase mimetic properties. Free Radic. Biol. Med. 2004, 37, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Grebinyk, A.; Prylutska, S.; Grebinyk, S.; Prylutskyy, Y.; Ritter, U.; Matyshevska, O.; Dandekar, T.; Frohme, M. Complexation with C60 Fullerene Increases Doxorubicin Efficiency against Leukemic Cells In Vitro. Nanoscale Res. Lett. 2019, 14, 61. [Google Scholar] [CrossRef]
- Yu, C.; Avci, P.; Canteenwala, T.; Chiang, L.Y.; Chen, B.J.; Hamblin, M.R. Photodynamic Therapy with Hexa(sulfo-n-butyl)[60]Fullerene Against Sarcoma In Vitro and In Vivo. J. Nanosci. Nanotechnol. 2016, 16, 171–181. [Google Scholar] [CrossRef]
- Liao, F.; Saitoh, Y.; Miwa, N. Anticancer Effects of Fullerene [C60] Included in Polyethylene Glycol Combined With Visible Light Irradiation Through ROS Generation and DNA Fragmentation on Fibrosarcoma Cells With Scarce Cytotoxicity to Normal Fibroblasts. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2011, 19, 203–216. [Google Scholar] [CrossRef]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar] [CrossRef]
- Myhre, O.; Andersen, J.M.; Aarnes, H.; Fonnum, F. Evaluation of the probes 2’,7’-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem. Pharmacol. 2003, 65, 1575–1582. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one-photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Fesik, S.W. Promoting apoptosis as a strategy for cancer drug discovery. Nat. Rev. Cancer 2005, 5, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Denning, D.P.; Imanishi, E.; Horvitz, H.R.; Nagata, S. Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science 2013, 341, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Fojtu, M.; Gumulec, J.; Stracina, T.; Raudenska, M.; Skotakova, A.; Vaculovicova, M.; Adam, V.; Babula, P.; Novakova, M.; Masarik, M. Reduction of Doxorubicin-Induced Cardiotoxicity Using Nanocarriers: A Review. Curr. Drug Metab. 2017, 18, 237–263. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.R.; Guhagarkar, S.A.; Devarajan, P.V. Engineered nanocarriers of doxorubicin: A current update. Crit. Rev. Ther. Drug Carr. Syst. 2008, 25, 1–61. [Google Scholar] [CrossRef]
- Bulavin, L.A.; Prylutskyy, Y.; Kyzyma, O.; Evstigneev, M.; Ritter, U.; Scharff, P. Self-Organization of Pristine C60 Fullerene and its Complexes with Chemotherapy Drugs in Aqueous Solution as Promising Anticancer Agents. In Modern Problems of Molecular Physics; Bulavin, L.A., Chalyi, A.V., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; Volume 197, pp. 3–22. [Google Scholar]
- Russ, K.A.; Elvati, P.; Parsonage, T.L.; Dews, A.; Jarvis, J.A.; Ray, M.; Schneider, B.; Smith, P.J.S.; Williamson, P.T.F.; Violi, A.; et al. C60 fullerene localization and membrane interactions in RAW 264.7 immortalized mouse macrophages. Nanoscale 2016, 8, 4134–4144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.W.; Yang, J.; Barron, A.R.; Monteiro-Riviere, N.A. Endocytic mechanisms and toxicity of a functionalized fullerene in human cells. Toxicol. Lett. 2009, 191, 149–157. [Google Scholar] [CrossRef]
- Asada, R.; Liao, F.; Saitoh, Y.; Miwa, N. Photodynamic anti-cancer effects of fullerene [C60]–PEG complex on fibrosarcomas preferentially over normal fibroblasts in terms of fullerene uptake and cytotoxicity. Mol. Cell. Biochem. 2014, 390, 175–184. [Google Scholar] [CrossRef]
- Prylutska, S.; Panchuk, R.; Gołuński, G.; Skivka, L.; Prylutskyy, Y.; Hurmach, V.; Skorohyd, N.; Borowik, A.; Woziwodzka, A.; Piosik, J.; et al. C60 fullerene enhances cisplatin anticancer activity and overcomes tumor cell drug resistance. Nano Res. 2017, 10, 652–671. [Google Scholar] [CrossRef]
- Franskevych, D.; Palyvoda, K.; Petukhov, D.; Prylutska, S.; Grynyuk, I.; Schuetze, C.; Drobot, L.; Matyshevska, O.; Ritter, U. Fullerene C60 Penetration into Leukemic Cells and Its Photoinduced Cytotoxic Effects. Nanoscale Res. Lett. 2017, 12, 40. [Google Scholar] [CrossRef]
- Yu, C.-H.; Lin, H.-P.; Chen, H.-M.; Yang, H.; Wang, Y.-P.; Chiang, C.-P. Comparison of clinical outcomes of oral erythroleukoplakia treated with photodynamic therapy using either light-emitting diode or laser light. Lasers Surg. Med. 2009, 41, 628–633. [Google Scholar] [CrossRef]
- Erkiert-Polguj, A.; Halbina, A.; Polak-Pacholczyk, I.; Rotsztejn, H. Light-emitting diodes in photodynamic therapy in non-melanoma skin cancers--own observations and literature review. J. Cosmet. Laser Ther. 2016, 18, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Hirshburg, J.; Choi, B.; Nelson, J.S.; Yeh, A.T. Correlation between collagen solubility and skin optical clearing using sugars. Lasers Surg. Med. 2007, 39, 140–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, D.; Larin, K.V.; Luo, Q.; Tuchin, V.V. Recent progress in tissue optical clearing. Laser Photon. Rev. 2013, 7, 732–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.J.; Ahn, Y.S.; Youn, Y.S.; Lee, E.S. Poly(ethylene glycol)-crosslinked fullerenes for high efficient phototherapy: Multimeric Fullerenes. Polym. Adv. Technol. 2013, 24, 220–227. [Google Scholar] [CrossRef]
- Yin, R.; Wang, M.; Huang, Y.-Y.; Huang, H.-C.; Avci, P.; Chiang, L.Y.; Hamblin, M.R. Photodynamic therapy with decacationic [60] fullerene monoadducts: Effect of a light absorbing electron-donor antenna and micellar formulation. Nanomedicine 2014, 10, 795–808. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, L.; Wei, S.; Ge, X.; Zhou, J.; Jiang, H.; Li, F.; Shen, J. Combination of chemotherapy and photodynamic therapy using graphene oxide as drug delivery system. J. Photochem. Photobiol. B Biol. 2014, 135, 7–16. [Google Scholar] [CrossRef]
- Khdair, A.; Chen, D.; Patil, Y.; Ma, L.; Dou, Q.P.; Shekhar, M.P.V.; Panyam, J. Nanoparticle-mediated combination chemotherapy and photodynamic therapy overcomes tumor drug resistance. J. Control Release 2010, 141, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Kalluru, P.; Vankayala, R.; Chiang, C.-S.; Hwang, K.C. Unprecedented “All-in-One” Lanthanide-Doped Mesoporous Silica Frameworks for Fluorescence/MR Imaging and Combination of NIR Light Triggered Chemo-Photodynamic Therapy of Tumors. Adv. Funct. Mater. 2016, 26, 7908–7920. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, X.; Wang, C.; Feng, L.; Li, Y.; Liu, Z. Drug-Induced Self-Assembly of Modified Albumins as Nano-theranostics for Tumor-Targeted Combination Therapy. ACS Nano 2015, 9, 5223–5233. [Google Scholar] [CrossRef]
- Arya, N.; Arora, A.; Vasu, K.S.; Sood, A.K.; Katti, D.S. Combination of single walled carbon nanotubes/graphene oxide with paclitaxel: A reactive oxygen species mediated synergism for treatment of lung cancer. Nanoscale 2013, 5, 2818–2829. [Google Scholar] [CrossRef]








| IC50, nM | Dark | 5 J/cm2 | 10 J/cm2 |
|---|---|---|---|
| Dox | 390 ± 56 | 382 ± 53 | 336 ± 49 |
| 1:1 C60-Dox | 135 ± 29 | 86 ± 19 | 44 ± 7 * |
| 2:1 C60-Dox | 225 ± 34 ** | 64 ± 11 * | 25 ± 4 *,** |
| CI | 5 J/cm2 | 10 J/cm2 |
|---|---|---|
| 1:1 C60-Dox | 0.546 ± 0.037 (synergism) | 0.130 ± 0.009 (strong synergism) |
| 2:1 C60-Dox | 0.316 ± 0.023 (synergism) | 0.097 ± 0.002 (very strong synergism) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grebinyk, A.; Prylutska, S.; Chepurna, O.; Grebinyk, S.; Prylutskyy, Y.; Ritter, U.; Ohulchanskyy, T.Y.; Matyshevska, O.; Dandekar, T.; Frohme, M. Synergy of Chemo- and Photodynamic Therapies with C60 Fullerene-Doxorubicin Nanocomplex. Nanomaterials 2019, 9, 1540. https://doi.org/10.3390/nano9111540
Grebinyk A, Prylutska S, Chepurna O, Grebinyk S, Prylutskyy Y, Ritter U, Ohulchanskyy TY, Matyshevska O, Dandekar T, Frohme M. Synergy of Chemo- and Photodynamic Therapies with C60 Fullerene-Doxorubicin Nanocomplex. Nanomaterials. 2019; 9(11):1540. https://doi.org/10.3390/nano9111540
Chicago/Turabian StyleGrebinyk, Anna, Svitlana Prylutska, Oksana Chepurna, Sergii Grebinyk, Yuriy Prylutskyy, Uwe Ritter, Tymish Y. Ohulchanskyy, Olga Matyshevska, Thomas Dandekar, and Marcus Frohme. 2019. "Synergy of Chemo- and Photodynamic Therapies with C60 Fullerene-Doxorubicin Nanocomplex" Nanomaterials 9, no. 11: 1540. https://doi.org/10.3390/nano9111540
APA StyleGrebinyk, A., Prylutska, S., Chepurna, O., Grebinyk, S., Prylutskyy, Y., Ritter, U., Ohulchanskyy, T. Y., Matyshevska, O., Dandekar, T., & Frohme, M. (2019). Synergy of Chemo- and Photodynamic Therapies with C60 Fullerene-Doxorubicin Nanocomplex. Nanomaterials, 9(11), 1540. https://doi.org/10.3390/nano9111540