Complexes Formed by Hydrophobic Interaction between Ag-Nanospheres and Adsorbents for the Detection of Methyl Salicylate VOC
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Description
2.2. Experimental Procedure
2.2.1. Fabrication of AgNSs
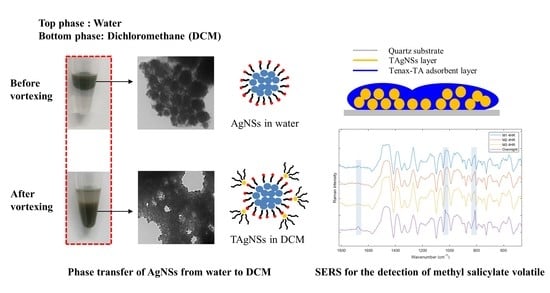
2.2.2. Phase Transfer
Cationic Surfactants in Aqueous Phase
Cationic Surfactants in Non-Aqueous Phase
2.2.3. 3D SERS Substrate
2.2.4. Static Volatile Collection
2.2.5. Characterization Analysis
2.2.6. UV/Vis Spectroscopy
2.2.7. Raman Spectroscopy
3. Results and Discussion
3.1. Phase Transfer of AgNSs
3.1.1. Cationic Surfactants in Aqueous Solution
3.1.2. Cationic Surfactants in Non-Aqueous Solution
3.2. SERS Application for the Determination of MeSA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mosier-Boss, P.A. Review of SERS substrates for chemical sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.; Jaatinen, E.; Buividas, R.; Seniutinas, G.; Juodkazis, S.; Izake, E.L.; Fredericks, P.M. SERS substrate for detection of explosives. Nanoscale 2012, 4, 7419–7424. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Lee, Y.H.; Koh, C.S.L.; Phan-Quang, G.C.; Han, X.; Lay, C.L.; Sim, H.Y.F.; Kao, Y.-C.; An, Q.; Ling, X.Y. Designing surface-enhanced Raman scattering (SERS) platforms beyond hotspot engineering: Emerging opportunities in analyte manipulations and hybrid materials. Chem. Soc. Rev. 2019, 48, 731–756. [Google Scholar] [CrossRef] [PubMed]
- Kuttner, C. Plasmonics in Sensing: From Colorimetry to SERS Analytics. In Plasmonics; IntechOpen: London, UK, 2018. [Google Scholar]
- Xu, H.; Aizpurua, J.; Käll, M.; Apell, P. Electromagnetic contributions to single-molecule sensitivity in surface-enhanced Raman scattering. Phys. Rev. E 2000, 62, 4318. [Google Scholar] [CrossRef]
- Jiang, X.; Lai, Y.; Yang, M.; Yang, H.; Jiang, W.; Zhan, J. Silver nanoparticle aggregates on copper foil for reliable quantitative SERS analysis of polycyclic aromatic hydrocarbons with a portable Raman spectrometer. Analyst 2012, 137, 3995–4000. [Google Scholar] [CrossRef]
- Chen, J.; Qin, G.; Wang, J.; Yu, J.; Shen, B.; Li, S.; Ren, Y.; Zuo, L.; Shen, W.; Das, B. One-step fabrication of sub-10-nm plasmonic nanogaps for reliable SERS sensing of microorganisms. Biosens. Bioelectron. 2013, 44, 191–197. [Google Scholar] [CrossRef]
- Tang, H.; Meng, G.; Huang, Q.; Zhang, Z.; Huang, Z.; Zhu, C. Arrays of Cone-Shaped ZnO Nanorods Decorated with Ag Nanoparticles as 3D Surface-Enhanced Raman Scattering Substrates for Rapid Detection of Trace Polychlorinated Biphenyls. Adv. Funct. Mater. 2012, 22, 218–224. [Google Scholar] [CrossRef]
- Li, Z.; Meng, G.; Huang, Q.; Hu, X.; He, X.; Tang, H.; Wang, Z.; Li, F. Ag Nanoparticle-Grafted PAN-Nanohump Array Films with 3D High-Density Hot Spots as Flexible and Reliable SERS Substrates. Small 2015, 11, 5452–5459. [Google Scholar] [CrossRef]
- Zhang, Q.; Lee, Y.H.; Phang, I.Y.; Lee, C.K.; Ling, X.Y. Hierarchical 3D SERS Substrates Fabricated by Integrating Photolithographic Microstructures and Self-Assembly of Silver Nanoparticles. Small 2014, 10, 2703–2711. [Google Scholar] [CrossRef]
- Bechelany, M.; Brodard, P.; Philippe, L.; Michler, J. Extended domains of organized nanorings of silver grains as surface-enhanced Raman scattering sensors for molecular detection. Nanotechnology 2009, 20, 455302. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.-D.; Tian, T.; Chu, L.-Q. Incorporation of multilayered silver nanoparticles into polymer brushes as 3-dimensional SERS substrates and their application for bacteria detection. Appl. Surf. Sci. 2017, 407, 185–191. [Google Scholar] [CrossRef]
- Cho, W.J.; Kim, Y.; Kim, J.K. Ultrahigh-density array of silver nanoclusters for SERS substrate with high sensitivity and excellent reproducibility. ACS Nano 2011, 6, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Senapati, D.; Khan, S.A.; Singh, A.K.; Hamme, A.; Yust, B.; Sardar, D.; Ray, P.C. Popcorn-Shaped Magnetic Core–Plasmonic Shell Multifunctional Nanoparticles for the Targeted Magnetic Separation and Enrichment, Label-Free SERS Imaging, and Photothermal Destruction of Multidrug-Resistant Bacteria. Chem. A Eur. J. 2013, 19, 2839–2847. [Google Scholar] [CrossRef] [PubMed]
- Yap, F.L.; Thoniyot, P.; Krishnan, S.; Krishnamoorthy, S. Nanoparticle cluster arrays for high-performance SERS through directed self-assembly on flat substrates and on optical fibers. Acs Nano 2012, 6, 2056–2070. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, J.; Peng, Q.; Li, Y. A general strategy for nanocrystal synthesis. Nature 2005, 437, 121. [Google Scholar] [CrossRef]
- Bai, F.; Wang, D.; Huo, Z.; Chen, W.; Liu, L.; Liang, X.; Chen, C.; Wang, X.; Peng, Q.; Li, Y. A Versatile Bottom-up Assembly Approach to Colloidal Spheres from Nanocrystals. Angew. Chem. Int. Ed. 2007, 46, 6650–6653. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, K.; Irudayaraj, J. Silver nanosphere SERS probes for sensitive identification of pathogens. J. Phys. Chem. C 2010, 114, 16122–16128. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Ding, H.; Xu, S.; Li, M.; Kong, F.; Luo, Y.; Li, G. Self-assembly of noble metallic spherical aggregates from monodisperse nanoparticles: Their synthesis and pronounced SERS and catalytic properties. J. Mater. Chem. A 2013, 1, 3362–3371. [Google Scholar] [CrossRef]
- Lee, K.-M.; Herrman, T.J.; Bisrat, Y.; Murray, S.C. Feasibility of surface-enhanced raman spectroscopy for rapid detection of aflatoxins in maize. J. Agric. Food Chem. 2014, 62, 4466–4474. [Google Scholar] [CrossRef]
- Lee, K.-M.; Herrman, T.J. Determination and prediction of fumonisin contamination in maize by surface–enhanced Raman spectroscopy (SERS). Food Bioprocess Technol. 2016, 9, 588–603. [Google Scholar] [CrossRef]
- Han, Z.; Liu, H.; Wang, B.; Weng, S.; Yang, L.; Liu, J. Three-dimensional surface-enhanced Raman scattering hotspots in spherical colloidal superstructure for identification and detection of drugs in human urine. Anal. Chem. 2015, 87, 4821–4828. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, J.Y.; Ying, J.Y. Phase transfer and its applications in nanotechnology. Chem. Soc. Rev. 2011, 40, 1672–1696. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mukherjee, P.; Guha, A.; Adyantaya, S.; Mandale, A.; Kumar, R.; Sastry, M. Amphoterization of colloidal gold particles by capping with valine molecules and their phase transfer from water to toluene by electrostatic coordination with fatty amine molecules. Langmuir 2000, 16, 9775–9783. [Google Scholar] [CrossRef]
- Mayya, K.S.; Caruso, F. Phase transfer of surface-modified gold nanoparticles by hydrophobization with alkylamines. Langmuir 2003, 19, 6987–6993. [Google Scholar] [CrossRef]
- Yao, H.; Momozawa, O.; Hamatani, T.; Kimura, K. Phase transfer of gold nanoparticles across a water/oil interface by stoichiometric ion-pair formation on particle surfaces. Bull. Chem. Soc. Jpn. 2000, 73, 2675–2678. [Google Scholar] [CrossRef]
- Yao, H.; Momozawa, O.; Hamatani, T.; Kimura, K. Stepwise size-selective extraction of carboxylate-modified gold nanoparticles from an aqueous suspension into toluene with tetraoctylammonium cations. Chem. Mater. 2001, 13, 4692–4697. [Google Scholar] [CrossRef]
- Devarajan, S.; Vimalan, B.; Sampath, S. Phase transfer of Au–Ag alloy nanoparticles from aqueous medium to an organic solvent: Effect of aging of surfactant on the formation of Ag-rich alloy compositions. J. Colloid Interface Sci. 2004, 278, 126–132. [Google Scholar] [CrossRef]
- Cheng, H.-W.; Schadt, M.J.; Young, K.; Luo, J.; Zhong, C.-J. Determination of ion pairing on capping structures of gold nanoparticles by phase extraction. Analyst 2015, 140, 6239–6244. [Google Scholar] [CrossRef]
- Cheng, H.-W.; Schadt, M.J.; Zhong, C.-J. Titration of gold nanoparticles in phase extraction. Analyst 2015, 140, 8023–8032. [Google Scholar] [CrossRef]
- Guo, H.; Xing, B.; White, J.C.; Mukherjee, A.; He, L. Ultra-sensitive determination of silver nanoparticles by surface-enhanced Raman spectroscopy (SERS) after hydrophobization-mediated extraction. Analyst 2016, 141, 5261–5264. [Google Scholar] [CrossRef]
- Yao, Q.; Yuan, X.; Yu, Y.; Yu, Y.; Xie, J.; Lee, J.Y. Introducing amphiphilicity to noble metal nanoclusters via phase-transfer driven ion-pairing reaction. J. Am. Chem. Soc. 2015, 137, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Luo, Z.; Zhang, Q.; Zhang, X.; Zheng, Y.; Lee, J.Y.; Xie, J. Synthesis of highly fluorescent metal (Ag, Au, Pt, and Cu) nanoclusters by electrostatically induced reversible phase transfer. ACS Nano 2011, 5, 8800–8808. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, A.M.; Morozova, J.E.; Shalaeva, Y.V.; Syakaev, V.V.; Nizameev, I.R.; Kadirov, M.K.; Antipin, I.S.; Konovalov, A.I. The supramolecular approach to the phase transfer of carboxylic calixresorcinarene-capped silver nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2017, 524, 127–134. [Google Scholar] [CrossRef]
- Liu, J.; Alvarez, J.; Ong, W.; Román, E.; Kaifer, A.E. Phase transfer of hydrophilic, cyclodextrin-modified gold nanoparticles to chloroform solutions. J. Am. Chem. Soc. 2001, 123, 11148–11154. [Google Scholar] [CrossRef]
- Sapoletova, N.A.; Kushnir, S.E.; Kushnir, A.E.; Kocherginskaya, P.B.; Kazin, P.E.; Napolskii, K.S. Simple phase transfer of nanoparticles from aqueous to organic media using polymer colloids as carriers. RSC Adv. 2016, 6, 112409–112412. [Google Scholar] [CrossRef]
- Park, J.; Thomasson, J.A.; Lee, K.-M. Volatile Detection from Plant Headspace with Modified Surface-Enhanced Raman Spectroscopy. In Proceedings of the 2017 ASABE Annual International Meeting, Spokane, WA, USA, 16–19 July 2017; p. 1. [Google Scholar]
- Kazakova, J.; García-Povea, A.; Fernández-Palacios, M.; Villar-Navarro, M.; Carnerero, J.M.; Jimenez-Ruiz, A.; Prado-Gotor, R. A colorimetric study of the interaction of cationic and anionic surfactants with anionic gold nanoparticles. Colloid Polym. Sci. 2017, 295, 2141–2149. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, E. Size-dependent phase transfer of gold nanoparticles from water into toluene by tetraoctylammonium cations: A wholly electrostatic interaction. J. Phys. Chem. B 2004, 108, 24–26. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20. [Google Scholar] [CrossRef]
- Park, J.-W.; Shumaker-Parry, J.S. Structural study of citrate layers on gold nanoparticles: Role of intermolecular interactions in stabilizing nanoparticles. J. Am. Chem. Soc. 2014, 136, 1907–1921. [Google Scholar] [CrossRef]
- De Aguiar, H.B.; Strader, M.L.; de Beer, A.G.; Roke, S. Surface structure of sodium dodecyl sulfate surfactant and oil at the oil-in-water droplet liquid/liquid interface: A manifestation of a nonequilibrium surface state. J. Phys. Chem. B 2011, 115, 2970–2978. [Google Scholar] [CrossRef]
- Navarro, J.R.; Werts, M.H. Resonant light scattering spectroscopy of gold, silver and gold–silver alloy nanoparticles and optical detection in microfluidic channels. Analyst 2013, 138, 583–592. [Google Scholar] [CrossRef]
- Park, S.-W.; Kaimoyo, E.; Kumar, D.; Mosher, S.; Klessig, D.F. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 2007, 318, 113–116. [Google Scholar] [CrossRef]
- Alfeeli, B.; Jain, V.; Johnson, R.K.; Beyer, F.L.; Heflin, J.R.; Agah, M. Characterization of poly (2, 6-diphenyl-p-phenylene oxide) films as adsorbent for microfabricated preconcentrators. Microchem. J. 2011, 98, 240–245. [Google Scholar] [CrossRef]
- Chae, M.-S.; Kim, J.; Yoo, Y.K.; Kang, J.Y.; Lee, J.H.; Hwang, K.S. A micro-preconcentrator combined olfactory sensing system with a micromechanical cantilever sensor for detecting 2, 4-dinitrotoluene gas vapor. Sensors 2015, 15, 18167–18177. [Google Scholar] [CrossRef] [Green Version]
- Drenscko, M.; Loverde, S.M. Molecular dynamics simulations of the interaction of phospholipid bilayers with polycaprolactone. Mol. Simul. 2019, 45, 859–867. [Google Scholar] [CrossRef]
- Berret, J.-F.; Yokota, K.; Morvan, M.; Schweins, R. Polymer− nanoparticle complexes: From dilute solution to solid state. J. Phys. Chem. B 2006, 110, 19140–19146. [Google Scholar] [CrossRef]
- Varghese, H.T.; Panicker, C.Y.; Philip, D.; Mannekutla, J.R.; Inamdar, S. IR, Raman and SERS studies of methyl salicylate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 66, 959–963. [Google Scholar] [CrossRef]
- Kuttner, C.; Höller, R.P.; Quintanilla, M.; Schnepf, M.J.; Dulle, M.; Fery, A.; Liz-Marzán, L.M. SERS and plasmonic heating efficiency from anisotropic core/satellite superstructures. Nanoscale 2019, 11, 17655–17663. [Google Scholar] [CrossRef] [Green Version]
- Xia, D.; Guo, Q.H.; Ge, M.; Yuan, Y.X.; Xu, M.M.; Yao, J.L. On-line sensitive detection of aromatic vapor through PDMS/C3H7S-assisted SERS amplification. RSC Adv. 2016, 6, 53289–53295. [Google Scholar] [CrossRef]
- Li, M.; Qiu, Y.; Fan, C.; Cui, K.; Zhang, Y.; Xiao, Z. Design of SERS nanoprobes for Raman imaging: Materials, critical factors and architectures. Acta Pharm. Sin. B 2018, 8, 381–389. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Thomasson, J.A.; Fernando, S.; Lee, K.-M.; Herrman, T.J. Complexes Formed by Hydrophobic Interaction between Ag-Nanospheres and Adsorbents for the Detection of Methyl Salicylate VOC. Nanomaterials 2019, 9, 1621. https://doi.org/10.3390/nano9111621
Park J, Thomasson JA, Fernando S, Lee K-M, Herrman TJ. Complexes Formed by Hydrophobic Interaction between Ag-Nanospheres and Adsorbents for the Detection of Methyl Salicylate VOC. Nanomaterials. 2019; 9(11):1621. https://doi.org/10.3390/nano9111621
Chicago/Turabian StylePark, Jinhyuk, J. Alex Thomasson, Sandun Fernando, Kyung-Min Lee, and Timothy J. Herrman. 2019. "Complexes Formed by Hydrophobic Interaction between Ag-Nanospheres and Adsorbents for the Detection of Methyl Salicylate VOC" Nanomaterials 9, no. 11: 1621. https://doi.org/10.3390/nano9111621
APA StylePark, J., Thomasson, J. A., Fernando, S., Lee, K. -M., & Herrman, T. J. (2019). Complexes Formed by Hydrophobic Interaction between Ag-Nanospheres and Adsorbents for the Detection of Methyl Salicylate VOC. Nanomaterials, 9(11), 1621. https://doi.org/10.3390/nano9111621