1. Introduction
Pb-based hybrid perovskites (Pb perovskites) solar cells have several advantages, such as remarkable device efficiency, low-cost fabrication, and unique optoelectronic properties [
1,
2,
3]. Thus, these are now considered to be the most promising substitute for Si, widely used in the solar market. However, several challenges, such as scale-up and reproducibility issues in performance and the manufacturing process, as well as toxicity, must be solved to ensure success in the commercial market. Therefore, considerable efforts are being made to address these issues, or to develop tandem solar cells combined with other solar cells [
1]. Researchers are also constantly looking for alternatives to Pb perovskites [
4].
Recently, Bi-based chalcohalides, such as BiSI and BiSeI, are being considered as good alternatives because of their suitability as solar absorbers and stability [
4,
5,
6,
7,
8,
9,
10]. In addition, these materials have a lower material cost compared to Pb perovskites (
Supplementary Table S1) and low toxicity, due to non-toxic Bi content [
5]. In particular, the solar cells based on them are expected to exhibit a high device efficiency because of the ns
2 electronic configuration of Bi
3+ (like Pb
2+ of Pb perovskites), enabling defect-tolerant features [
4,
5,
6]. Despite their great potential, little work has been carried out on solar cells, and their best reported device efficiency of 1.32% is unsatisfactory [
8,
9]. Therefore, further work is required to prove their potential as a photovoltaic material. However, fabrication methods suitable for solar cells are still lacking and have not been optimized to achieve the best performance.
BiSI and BiSeI have been fabricated using several methods, including a spray solution method [
8], conversion reaction from BiOI particles under H
2(S,Se) gas [
10], and a single source precursor solution method [
9]. These methods are simple, versatile, and cost-effective because they are based on a solution process and performed at a low temperature below 300 °C. However, these techniques have not been fully validated as suitable methods for solar cells. Therefore, it is still necessary to develop an approach that can control the key variables, such as the crystal structure, morphology, and optoelectronic properties. We recently developed a simple solution method for typical Sb chalcohalides, SbSI, via a two-step process [
11]: (i) formation of Sb
2S
3 and (ii) its conversion to SbSI based on the simple chemical reaction of Sb
2S
3 + SbI
3 → 3SbSI. With this method, the structure and morphology of SbSI were successfully controlled by tuning experimental parameters at each step. Given that SbSI is isostructural to BiSI [
12], this two-step method can be easily applied to the BiSI fabrication by the following chemical reaction: Bi
2S
3 + BiI
3 → 3BiSI. To this end, we chose the thiol-amine solution method for the Bi
2S
3 fabrication in step I, which turned out to be a very effective approach of preparing various chalcogenides [
13,
14].
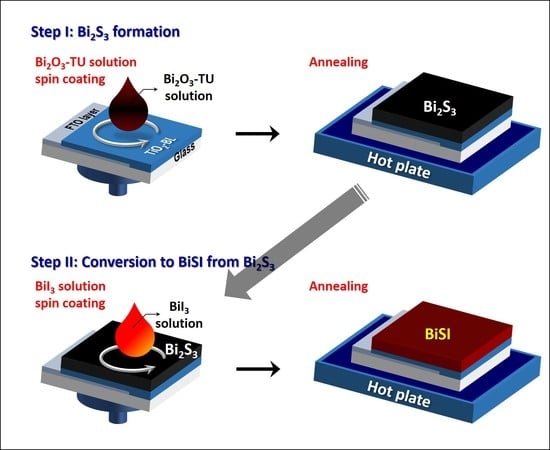
In this work, we introduce the fabrication of BiSI films via our two-step solution process for solar cell applications. The Bi2S3 is first fabricated using the Bi2O3-thiourea (Bi2O3-TU) thiol-amine solution (step I), where Bi2O3 and TU are dissolved in 2-mercaptoethanol/ethanolamine (1/4 v/v) mixture. Then, it is converted to BiSI in the chemical reaction between as-formed Bi2S3 and BiI3 (step II). The structures, absorption, and morphology are controlled by tuning the Bi:S molar ratio of the Bi2O3-TU solution and the number of repetitions of step I. The morphology of as-fabricated BiSI film exhibits nanorods rather than a compact film. In addition, the electronic structure of BiSI is investigated and discussed for solar cell applications.
3. Results and Discussion
Figure 1a shows the schematic diagram of the BiSI fabrication process. The samples fabricated after step I and II exhibit orthorhombic Bi
2S
3 (reference code: 98-061-7028) and orthorhombic BiSI (reference code: 98-002-3631) phases, respectively, as shown in
Figure 1b. The optical band gaps
EG of Bi
2S
3 and BiSI are measured as 1.57 eV and 1.61 eV, respectively. These are close to the reported values of 1.3–1.7 [
16,
17] and 1.5–1.8 eV [
5,
6,
7,
8,
9,
18,
19], respectively. They have similar absorption edges around 1.6 eV (
Figure 1c), thereby showing a similar sample color, as shown in the inset image. These results indicate that BiSI film was successfully obtained from the Bi
2S
3 film at a low temperature of 200 °C via our two-step solution process.
We found that the Bi:S molar ratio of the Bi
2O
3-TU solution, used in step I, strongly affects the BiSI formation. We controlled the molar ratio of the solution based on the stoichiometric molar fraction of Bi/S = 1/1.5 for Bi
2S
3.
Figure 2a shows XRD patterns of the BiSI thin films fabricated with the same conditions as the sample shown in
Figure 1 but using Bi
2O
3-TU solution of different Bi:S molar ratios. All samples exhibit a pure orthorhombic BiSI phase but show a different intensity for each peak. To clearly display the difference in peak intensity of each pattern, we highlighted two peaks corresponding to (102) and (200) planes, shown in the inset image. The intensity of the two peaks gradually increased as the amount of S increased in Bi:S, from 1:1 to 1:3, and then decreased at Bi:S = 1:4. The absorption spectra (
Figure 2b) exhibited similar trends, where the absorption edges were the same regardless of the ratio and consistent with the value of
Figure 1c. These results indicate that the Bi:S ratio plays a key role in BiSI formation.
The different BiSI formation by the Bi:S molar ratio may be explained in terms of the contribution of excess TU to BiSI formation. The TU as a sulfur source should be sufficiently supplied at each step to compensate for the loss of volatile TU at our annealing temperature of 200 °C. In addition, an excess TU may contribute to stabilizing the Bi-TU complex in the solution [
11,
20], enabling a stable supply of S. Thus, the BiSI formation is more enhanced as the more TU is supplied as shown in
Figure 2. However, at a highly excess TU condition, organic residues derived from residual TU may interfere with BiSI formation. As a result, the BiSI with maximum intensity can be obtained at the specific ratio of Bi:S = 1:3. Interestingly, the optimum ratio of Bi:S (1:3) is same as that of Sb:S used for SbSI [
11], although the solution method and the material are quite different. Besides, the TU plays a similar role in forming the crystalline phase in both materials. Therefore, we can expect that the optimum ratio condition may be useful in the formation of other Sb/Bi chalcohalides, such as SbSeI and BiSeI.
The BiSI formation could also be controlled by adjusting the repetition of step I. The samples were fabricated under the same conditions as the sample of
Figure 1, except for the number of repetitions of step I. As shown in
Figure 3a,b, and
Supplementary Figure S2, Bi
2S
3 bundles consisting of nanorods with 10–30 nm diameters, were formed after step I and converted to BiSI nanorods in step II. The diameter (60–100 nm) of BiSI nanorods were larger than that of the Bi
2S
3 nanorods, suggesting that the Bi
2S
3 aggregates to form BiSI nanorods during step II. These results indicate that the BiSI film consists of nanorods rather than a continuous film. As the number of repetitions increased, the number of Bi
2S
3 bundles increased, as shown in
Figure 3a. As a result, more BiSI nanorods were formed in step II. Simultaneously, the thickness of the BiSI nanorod film was increased from 210 to 657 nm (
Supplementary Table S2). Thus, a large number of nanorods covered the entire surface after three repetitions (
Figure 3b and
Supplementary Figure S3). This increased number of nanorods was supported by enhanced absorption with increasing repetitions (
Figure 3c). To further confirm this, structures were investigated with XRD. The results are shown in
Figure 3d. Up to three repetitions (I-#3), the peak intensity of BiSI phase increased, but decreased when step I was repeated four times (I-#4). Simultaneously, the Bi
2S
3 phase appeared at I-#3 and its intensity was further enhanced at I-#4. This result indicates that some of Bi
2S
3 remained unconverted to BiSI in the two samples for I-#3 and I-#4. Thus, improved absorption observed at I-#3 and I-#4 can be explained by the contribution of BiSI and Bi
2S
3 rather than just BiSI, because both have similar absorption edges as shown in
Figure 1c. Therefore, pure-phase BiSI with maximum intensity could be obtained when step I was repeated twice.
Note that the repetition of step II did not cause further conversion of Bi
2S
3 to BiSI, formed in the I-#3 and I-#4 samples, but resulted in BiOI formation (
Supplementary Figure S4). To figure out the reason why BiSI was no longer converted from the Bi
2S
3 in I-#3 and I-#4, we examined their morphology and compared it with those obtained from the I-#1 and I-#2 samples. As show in
Figure 3a and
Supplementary Figure S2, the Bi
2S
3 bundles formed after step I, in I-#1 and I-#2, did not cover the entire surface of underlying TiO
2-BL, revealing a portion of the TiO
2-BL surface. The converted samples show the nanorods randomly grown on the TiO
2-BL/FTO substrate (
Figure 4a). As a result, the nanorods were grown from 210 to 410 nm as step I was repeated (
Table S2). In contrast, the Bi
2S
3 bundles were intricately intertwined so that the underlying TiO
2-BL surface was not revealed in I-#3 and I-#4 (
Figure 3a). The samples obtained from such intertwined bundles exhibit a two-layered structure, with nanorods on the upper layer and aggregated nanostructures on the lower layer (
Figure 4b). The thickness of each layer is very similar in I-#3 and I-#4 samples, as revealed in
Table S2, suggesting that the multiple coating did not affect the BiSI growth. By comparing morphologies and structures, it can be inferred that the nanorods in the upper layer and nanostructures in the lower layer mainly consisted of BiSI and Bi
2S
3, respectively. This was confirmed by the grazing incident XRD (GIXRD) measurement (
Supplementary Figure S5). These results indicate that the intertwined Bi
2S
3 bundles prevented the BiI
3 solution from reaching the bottom of the Bi
2S
3 layer, in the I-#3 and I-#4 samples. Thus, the Bi
2S
3 at the bottom could react with BiI
3, leaving unconverted Bi
2S
3 at the bottom. As a result, the BiSI nanorods were formed in the upper layer, in which the BiI
3 solution can actively react, whereas the nanostructures mainly composed of Bi
2S
3 are formed in the lower layer.
To investigate the electronic structure of the BiSI thin film, we measured ultraviolet photoemission spectroscopy (UPS) spectra, as shown in
Figure 5a. From the two spectra of the cutoff and valence band edge regions, we could obtain the cutoff energy
Ecutoff and difference Δ =
EV -
EF; where
EV is the valence band maximum and
EF is Fermi level. These were 16.7 and 1.3 eV, respectively. Correspondingly, we could calculate
EF,
EV, and conduction band minimum (CBM,
EC), which were 4.5, 5.9, and 4.3 eV, respectively, based on the relation of
EF = hν – (
Ecutoff − EVAC) (
EVAC: vacuum level energy) and
EG. From these results, it was revealed that the BiSI behaves like a n-type semiconductor, consistent with a previous study [
8], because
EF is located close to
EC. Based on this measurement, we drew an energy-level diagram for the as-fabricated BiSI nanorods film, as shown in
Figure 5b. We also included other widely used layers to examine its applicability to solar cells. For a solar cell that constructed with FTO, TiO
2, poly(3-hexylthiophene) (P3HT) as the conducting oxide, electron transporting layer (ETL), and hole transporting material (HTM), respectively, we expected very poor device performance. A preliminary result confirmed that this device did not work properly (
Supplementary Figure S6 and Table S2). These are for the following two reasons: First, it is very difficult for electrons to transfer from the CBM of BiSI to the CBM of TiO
2 because the CBM of BiSI is located below that of TiO
2. Second, the highest occupied molecular orbitals (HOMO) level of P3HT is located at 5.1 eV; ~0.6 eV lower than
EF of BiSI. Thus, the maximum open circuit voltage
VOC is limited to 0.6 eV. Therefore, to achieve a high device efficiency, these two issues must be solved.
The first issue can be addressed by using an alternative ETL that has a CBM lower than that of BiSI. Material engineering via doping/alloying for shifting the CBM of BiSI could be another solution for solving this, while maintaining the TiO
2 ETL. To address the second issue, a HTM with a deep HOMO level should be used, such as 3,3’-dimethoxybenzidine moieties (S9, 5.5 eV) [
21], poly(9,9-di-n-octylfluorenyl-2,7-diyl) (F8, 5.8 eV) [
5,
9] and poly(9,9-dioctylfluorene-alt-benzothiadiazole) (F8BT, 5.9 eV) [
22]. Very recently, Tiwari et al. addressed these issues by using SnO
2 and F8 as the ETL and HTM, respectively, and achieved the best device efficiency (1.32%) in BiSI solar cells [
9]. This efficiency is a significant improvement compared to 0.012% for the solar cell fabricated by Hahn et al [
8]. Despite great progress in performance, the best efficiency is still very far below that of Pb perovskites solar cells. Therefore, several other factors, such as defects, crystalline orientation, device architecture, and material engineering, should also be considered to further enhance the performance [
5,
6].