Simultaneous Removal and Recovery of Metal Ions and Dyes from Wastewater through Montmorillonite Clay Mineral
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples Preparation
2.3. X-ray Diffraction (XRD) Characterization
3. Results
3.1. Effect of pH Solution on Adsorption Efficiency
3.2. Adsorption Isotherms
3.3. XRD Characterization
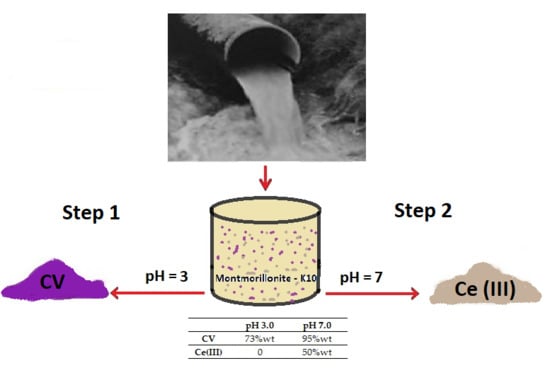
3.4. Protocol for the Removal and Separation of Pollutants
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Camera-Roda, G.; Loddo, V.; Palmisano, L.; Parrino, F. Photocatalytic ozonation for a sustainable aquaculture: A long-term test in a seawater aquarium. Appl. Catal. B Environ. 2019, 253, 69–76. [Google Scholar] [CrossRef]
- Toledano Garcia, D.; Ozer, L.Y.; Parrino, F.; Ahmed, M.; Brudecki, G.P.; Hasan, S.W.; Palmisano, G. Photocatalytic ozonation under visible light for the remediation of water effluents and its integration with an electro-membrane bioreactor. Chemosphere 2018, 209, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Neagu, S.; Cojoc, R.; Enache, M.; Mocioiu, O.C.; Precupas, A.; Popa, V.T.; Gomoiu, I.; Enache, M. Biotransformation of Waste Glycerol from Biodiesel Industry in Carotenoids Compounds by Halophilic Microorganisms. Waste Biomass Valorization 2019, 10, 45–52. [Google Scholar] [CrossRef]
- Matei, E.; Covaliu, C.I.; Predescu, A.; Berbecaru, A.; Tarcea, C.; Predescu, C. Remediation of Wastewater with Ultraviolet Irradiation Using a Novel Titanium (IV) Oxide Photocatalyst. Anal. Lett. 2019, 52, 2180–2187. [Google Scholar] [CrossRef]
- Cataldo, S.; Cavallaro, G.; Gianguzza, A.; Lazzara, G.; Pettignano, A.; Piazzese, D.; Villaescusa, I. Kinetic and equilibrium study for cadmium and copper removal from aqueous solutions by sorption onto mixed alginate/pectin gel beads. J. Environ. Chem. Eng. 2013, 1, 1252–1260. [Google Scholar] [CrossRef]
- Fancello, D.; Scalco, J.; Medas, D.; Rodeghero, E.; Martucci, A.; Meneghini, C.; De Giudici, G. XRD-Thermal Combined Analyses: An Approach to Evaluate the Potential of Phytoremediation, Phytomining, and Biochar Production. Int. J. Environ. Res. Public Health 2019, 16, 1976. [Google Scholar] [CrossRef] [PubMed]
- Leulescu, M.; Rotaru, A.; Pălărie, I.; Moanţă, A.; Cioateră, N.; Popescu, M.; Morîntale, E.; Bubulică, M.V.; Florian, G.; Hărăbor, A.; et al. Tartrazine: Physical, thermal and biophysical properties of the most widely employed synthetic yellow food-colouring azo dye. J. Therm. Anal. Calorim. 2018, 134, 209–231. [Google Scholar] [CrossRef]
- Politi, D.; Sidiras, D. Wastewater Treatment for Dyes and Heavy Metals Using Modified Pine Sawdust as Adsorbent. Procedia Eng. 2012, 42, 1969–1982. [Google Scholar] [CrossRef]
- Rotaru, A.; Dumitru, M. Thermal behaviour of CODA azoic dye liquid crystal and nanostructuring by drop cast and spin coating techniques. J. Therm. Anal. Calorim. 2017, 127, 21–32. [Google Scholar] [CrossRef]
- Varrica, D.; Dongarrà, G.; Alaimo, M.G.; Monna, F.; Losno, R.; Sanna, E.; De Giudici, G.; Tamburo, E. Lead isotopic fingerprint in human scalp hair: The case study of Iglesias mining district (Sardinia, Italy). Sci. Total Environ. 2018, 613–614, 456–461. [Google Scholar] [CrossRef]
- Visa, M.; Bogatu, C.; Duta, A. Simultaneous adsorption of dyes and heavy metals from multicomponent solutions using fly ash. Appl. Surf. Sci. 2010, 256, 5486–5491. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, Q.; Ren, X.; Yin, M.; Zhou, Y.; Xue, Z. Adsorption and removal of sulfonic dyes from aqueous solution onto a coordination polymeric xerogel with amino groups. Colloids Surf. Physicochem. Eng. Asp. 2015, 485, 125–135. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Giridhar, P.; Venugopalan, A.; Parimalan, R. A Review on Annatto Dye Extraction, Analysis and Processing—A Food Technology Perspective. J. Sci. Res. Rep. 2014, 3, 327–348. [Google Scholar] [CrossRef]
- Iqbal, M.; Nisar, J. Cytotoxicity and mutagenicity evaluation of gamma radiation and hydrogen peroxide treated textile effluents using bioassays. J. Environ. Chem. Eng. 2015, 3, 1912–1917. [Google Scholar] [CrossRef]
- Jain, R.; Sikarwar, S. Photocatalytic and Adsorption Studies on the Removal of Dye Congo Red from Wastewater. Int. J. Environ. Pollut. 2006, 27, 158–178. [Google Scholar] [CrossRef]
- Khan, N.; Kazi, T.G.; Tuzen, M.; Soylak, M. A multivariate study of solid phase extraction of beryllium(II) using human hair as adsorbent prior to its spectrophotometric detection. Desalin. Water Treat. 2015, 55, 1088–1095. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, C.H.; Han, S.H.; Suh, M.Y. Studies on complexation and solvent extraction of lanthanides in the presence of diaza-18-crown-6-di-isopropionic acid. Talanta 1997, 45, 437–444. [Google Scholar] [CrossRef]
- Parrino, F.; García-López, E.; Marcì, G.; Palmisano, L.; Felice, V.; Sora, I.N.; Armelao, L. Cu-substituted lanthanum ferrite perovskites: Preparation, characterization and photocatalytic activity in gas-solid regime under simulated solar light irradiation. J. Alloys Compd. 2016, 682, 686–694. [Google Scholar] [CrossRef]
- Seyahmazegi, E.N.; Mohammad-Rezaei, R.; Razmi, H. Multiwall carbon nanotubes decorated on calcined eggshell waste as a novel nano-sorbent: Application for anionic dye Congo red removal. Chem. Eng. Res. Des. 2016, 109, 824–834. [Google Scholar] [CrossRef]
- Shuai, A.; Xueyan, L.; Lijun, Y.; Lei, Z. Enhancement removal of crystal violet dye using magnetic calcium ferrite nanoparticle: Study in single- and binary-solute. Chem. Eng. Res. Des. 2015, 94, 726–735. [Google Scholar]
- Trudić, B.; Kebert, M.; Popovic, B.; Stajner, D.; Orlović, S.; Galović, V.; Pilipovic, A. The Effect of Heavy Metal Pollution in Soil on Serbian Poplar Clones. Sumar. List 2013, 137, 287–296. [Google Scholar]
- Wang, Y.; Xie, Y.; Zhang, Y.; Tang, S.; Guo, C.; Wu, J.; Lau, R. Anionic and cationic dyes adsorption on porous poly-melamine-formaldehyde polymer. Chem. Eng. Res. Des. 2016, 114, 258–267. [Google Scholar] [CrossRef]
- Xiong, C.; Liu, X.; Yao, C. Effect of pH on sorption for RE(III) and sorption behaviors of Sm(III) by D152 resin. J. Rare Earths 2008, 26, 851–856. [Google Scholar] [CrossRef]
- Bhatti, I.A.; Ahmad, N.; Iqbal, N.; Zahid, M.; Iqbal, M. Chromium adsorption using waste tire and conditions optimization by response surface methodology. J. Environ. Chem. Eng. 2017, 5, 2740–2751. [Google Scholar] [CrossRef]
- Bibi, I.; Nazar, N.; Iqbal, M.; Kamal, S.; Nawaz, H.; Nouren, S.; Safa, Y.; Jilani, K.; Sultan, M.; Ata, S.; et al. Green and eco-friendly synthesis of cobalt-oxide nanoparticle: Characterization and photo-catalytic activity. Adv. Powder Technol. 2017, 28, 2035–2043. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, M.; Hu, H.; Zhang, X. Mutagenicity and cytotoxicity assessment of biodegraded textile effluent by Ca-alginate encapsulated manganese peroxidase. Biochem. Eng. J. 2016, 109, 153–161. [Google Scholar] [CrossRef]
- Hussain, F.; Shah, S.Z.; Zhou, W.; Iqbal, M. Microalgae screening under CO2 stress: Growth and micro-nutrients removal efficiency. J. Photochem. Photobiol. B 2017, 170, 91–98. [Google Scholar] [CrossRef]
- Idrissi, M.; Miyah, Y.; Benjelloun, Y.; Chaouch, M. Degradation of crystal violet by heterogeneous Fenton-like reaction using Fe/Clay catalyst with H2O2. J. Mater. Environ. Sci. 2016, 7, 50–58. [Google Scholar]
- Kausar, A.; Bhatti, H.N.; Iqbal, M.; Ashraf, A. Batch versus column modes for the adsorption of radioactive metal onto rice husk waste: Conditions optimization through response surface methodology. Water Sci. Technol. 2017, 76, 1035–1043. [Google Scholar] [CrossRef]
- Shoukat, S.; Bhatti, H.N.; Iqbal, M.; Noreen, S. Mango stone biocomposite preparation and application for crystal violet adsorption: A mechanistic study. Microporous Mesoporous Mater. 2017, 239, 180–189. [Google Scholar] [CrossRef]
- Turabik, M. Adsorption of basic dyes from single and binary component systems onto bentonite: Simultaneous analysis of Basic Red 46 and Basic Yellow 28 by first order derivative spectrophotometric analysis method. J. Hazard. Mater. 2008, 158, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Ramachandran, M. Adsorptive removal of basic dyes from aqueous solutions by surfactant modified bentonite clay (organoclay): Kinetic and competitive adsorption isotherm. Process Saf. Environ. Prot. 2015, 95, 215–225. [Google Scholar] [CrossRef]
- Kausar, A.; Iqbal, M.; Javed, A.; Aftab, K.; Nazli, Z.-H.; Bhatti, H.N.; Nouren, S. Dyes adsorption using clay and modified clay: A review. J. Mol. Liq. 2018, 256, 395–407. [Google Scholar] [CrossRef]
- Toor, M.; Jin, B.; Dai, S.; Vimonses, V. Activating natural bentonite as a cost-effective adsorbent for removal of Congo-red in wastewater. J. Ind. Eng. Chem. 2015, 21, 653–661. [Google Scholar] [CrossRef]
- Wu, D.; Niu, C.; Li, D.; Bai, Y. Solvent extraction of scandium(III), yttrium(III), lanthanum(III) and gadolinium(III) using Cyanex 302 in heptane from hydrochloric acid solutions. J. Alloy. Compd. 2004, 374, 442–446. [Google Scholar] [CrossRef]
- Allen, S.J.; Mckay, G.; Porter, J.F. Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. J. Colloid Interface Sci. 2004, 280, 322–333. [Google Scholar] [CrossRef]
- Gupta, V.K.; Mittal, A.; Krishnan, L.; Gajbe, V. Adsorption kinetics and column operations for the removal and recovery of malachite green from wastewater using bottom ash. Sep. Purif. Technol. 2004, 40, 87–96. [Google Scholar] [CrossRef]
- Kamińska, G.; Dudziak, M.; Kudlek, E.; Bohdziewicz, J. Preparation, Characterization and Adsorption Potential of Grainy Halloysite-CNT Composites for Anthracene Removal from Aqueous Solution. Nanomaterials 2019, 9, 890. [Google Scholar] [CrossRef] [Green Version]
- Mittal, A.; Kurup (Krishnan), L.; Gupta, V.K. Use of waste materials—Bottom Ash and De-Oiled Soya, as potential adsorbents for the removal of Amaranth from aqueous solutions. J. Hazard. Mater. 2005, 117, 171–178. [Google Scholar] [CrossRef]
- Molino, A.; Erto, A.; Natale, F.D.; Donatelli, A.; Iovane, P.; Musmarra, D. Gasification of Granulated Scrap Tires for the Production of Syngas and a Low-Cost Adsorbent for Cd(II) Removal from Wastewaters. Ind. Eng. Chem. Res. 2013, 52, 12154–12160. [Google Scholar] [CrossRef]
- Sadraei, R.; Paganini, C.M.; Calza, P.; Magnacca, G. An Easy Synthesis for Preparing Bio-Based Hybrid Adsorbent Useful for Fast Adsorption of Polar Pollutants. Nanomaterials 2019, 9, 731. [Google Scholar] [CrossRef] [PubMed]
- Sert, Ş.; Kütahyali, C.; İnan, S.; Talip, Z.; Çetinkaya, B.; Eral, M. Biosorption of lanthanum and cerium from aqueous solutions by Platanus orientalis leaf powder. Hydrometallurgy 2008, 90, 13–18. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Balasubramanian, R. Single and binary biosorption of cerium and europium onto crab shell particles. Chem. Eng. J. 2010, 163, 337–343. [Google Scholar] [CrossRef]
- Walker, G.M.; Hansen, L.; Hanna, J.-A.; Allen, S.J. Kinetics of a reactive dye adsorption onto dolomitic sorbents. Water Res. 2003, 37, 2081–2089. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Removal of various pollutants from water and wastewater by modified chitosan adsorbents. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2331–2386. [Google Scholar] [CrossRef]
- XU, S.; Zhang, S.; Chen, K.; Han, J.; Liu, H.; Wu, K. Biosorption of La3+ and Ce3+ by Agrobacterium sp. HN1. J. Rare Earths 2011, 29, 265–270. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Almasri, D.A.; Saleh, N.B.; Atieh, M.A.; McKay, G.; Ahzi, S. Adsorption of phosphate on iron oxide doped halloysite nanotubes. Sci. Rep. 2019, 9, 3232. [Google Scholar] [CrossRef]
- Asere, T.G.; Stevens, C.V.; Laing, G.D. Use of (modified) natural adsorbents for arsenic remediation: A review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef]
- Celis, R.; HermosÍn, M.C.; Cornejo, J. Heavy Metal Adsorption by Functionalized Clays. Environ. Sci. Technol. 2000, 34, 4593–4599. [Google Scholar] [CrossRef]
- Erdem, E.; Karapinar, N.; Donat, R. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.K.; Panday, K.K.; Srivastava, R.M.; Singh, V.N. Removal of victoria blue from aqueous solution by fly ash. J. Chem. Technol. Biotechnol. 1987, 38, 99–104. [Google Scholar] [CrossRef]
- Kragović, M.; Pašalić, S.; Marković, M.; Petrović, M.; Nedeljković, B.; Momčilović, M.; Stojmenović, M. Natural and Modified Zeolite—Alginate Composites. Application for Removal of Heavy Metal Cations from Contaminated Water Solutions. Minerals 2018, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Ayoko, G.A.; Kurdi, R.; Horváth, E.; Kristóf, J.; Frost, R.L. Adsorption of phenolic compounds by organoclays: Implications for the removal of organic pollutants from aqueous media. J. Colloid Interface Sci. 2013, 406, 196–208. [Google Scholar] [CrossRef]
- Potgieter, J.H.; Potgieter-Vermaak, S.S.; Kalibantonga, P.D. Heavy metals removal from solution by palygorskite clay. Miner. Eng. 2006, 19, 463–470. [Google Scholar] [CrossRef]
- Rodeghero, E.; Chenet, T.; Martucci, A.; Ardit, M.; Sarti, E.; Pasti, L. Selective adsorption of toluene and n-hexane binary mixture from aqueous solution on zeolite ZSM-5: Evaluation of competitive behavior between aliphatic and aromatic compounds. Catal. Today 2019. [Google Scholar] [CrossRef]
- Stafiej, A.; Pyrzynska, K. Adsorption of heavy metal ions with carbon nanotubes. Sep. Purif. Technol. 2007, 58, 49–52. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Biopolymer-Targeted Adsorption onto Halloysite Nanotubes in Aqueous Media. Langmuir 2017, 33, 3317–3323. [Google Scholar] [CrossRef]
- Cataldo, S.; Lazzara, G.; Massaro, M.; Muratore, N.; Pettignano, A.; Riela, S. Functionalized halloysite nanotubes for enhanced removal of lead(II) ions from aqueous solutions. Appl. Clay Sci. 2018, 156, 87–95. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Sanzillo, V. Modified Halloysite Nanotubes: Nanoarchitectures for Enhancing the Capture of Oils from Vapor and Liquid Phases. ACS Appl. Mater. Interfaces 2014, 6, 606–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehsan, A.; Bhatti, H.N.; Iqbal, M.; Noreen, S. Native, acidic pre-treated and composite clay efficiency for the adsorption of dicationic dye in aqueous medium. Water Sci. Technol. 2016, 75, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Lvov, Y.; Aerov, A.; Fakhrullin, R. Clay nanotube encapsulation for functional biocomposites. Adv. Colloid Interface Sci. 2014, 207, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.; Bhatti, H.N.; Iqbal, M.; Noreen, S. Eriobotrya japonica seed biocomposite efficiency for copper adsorption: Isotherms, kinetics, thermodynamic and desorption studies. J. Environ. Manag. 2016, 176, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Abdullayev, E.; Vasiliev, A.; Lvov, Y. Halloysite nanotubule clay for efficient water purification. J. Colloid Interface Sci. 2013, 406, 121–129. [Google Scholar] [CrossRef]
- Santos, S.C.R.; Boaventura, R.A.R. Adsorption of cationic and anionic azo dyes on sepiolite clay: Equilibrium and kinetic studies in batch mode. J. Environ. Chem. Eng. 2016, 4, 1473–1483. [Google Scholar] [CrossRef]
- Baskaralingam, P.; Pulikesi, M.; Elango, D.; Ramamurthi, V.; Sivanesan, S. Adsorption of acid dye onto organobentonite. J. Hazard. Mater. 2006, 128, 138–144. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G.; Vayer, M. Chapter 12.10 Cation and Anion Exchange. In Developments in Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 979–1001. ISBN 1572-4352. [Google Scholar]
- Klika, Z.; Pustková, P.; Dudová, M.; Capkova, P.; Kliková, C.; Matys Grygar, T. The adsorption of methylene blue on montmorillonite from acid solutions. Clay Miner. 2011, 46, 461–471. [Google Scholar] [CrossRef]
- Vopálka, D.; Gondolli, J.; Drtinová, B.; Klika, Z. Cesium uptake by Ca/Mg bentonite: Evaluation of sorption experiments by a multicomponent two-site ion-exchange model. J. Radioanal. Nucl. Chem. 2015, 304, 429–434. [Google Scholar] [CrossRef]
- Calabrese, I.; Gelardi, G.; Merli, M.; Liveri, M.L.T.; Sciascia, L. Clay-biosurfactant materials as functional drug delivery systems: Slowing down effect in the in vitro release of cinnamic acid. Appl. Clay Sci. 2017, 135, 567–574. [Google Scholar] [CrossRef] [Green Version]
- Sciascia, L.; Turco Liveri, M.L.; Merli, M. Kinetic and equilibrium studies for the adsorption of acid nucleic bases onto K10 montmorillonite. Appl. Clay Sci. 2011, 53, 657–668. [Google Scholar] [CrossRef]
- Calabrese, I.; Cavallaro, G.; Scialabba, C.; Licciardi, M.; Merli, M.; Sciascia, L.; Liveri, M.L.T. Montmorillonite nanodevices for the colon metronidazole delivery. Int. J. Pharm. 2013, 457, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, I.; Gelardi, G.; Merli, M.; Ritwo, G.; Sciascia, L.; Liveri, M.L.T. New tailor-made bio-organoclays for the remediation of olive mill waste water. IOP Conf. Ser. Mater. Sci. Eng. 2013, 47, 012040. [Google Scholar] [CrossRef] [Green Version]
- Chaari, I.; Medhioub, M.; Jamoussi, F. Use of Clay to Remove Heavy Metals from Jebel Chakir Landfill Leachate. J. Appl. Sci. Environ. Sanit. 2011, 6, 143–148. [Google Scholar]
- Sharma, P.; Borah, D.J.; Das, P.; Das, M.R. Cationic and anionic dye removal from aqueous solution using montmorillonite clay: Evaluation of adsorption parameters and mechanism. Desalin. Water Treat. 2016, 57, 8372–8388. [Google Scholar] [CrossRef]
- Sciascia, L.; Casella, S.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Princivalle, F.; Parisi, F. Olive mill wastewaters decontamination based on organo-nano-clay composites. Ceram. Int. 2019, 45, 2751–2759. [Google Scholar] [CrossRef]
- Sarma, G.; Gupta, S.; Bhattacharyya, K. Adsorption of Crystal violet on raw and acid-treated montmorillonite, K10, in aqueous suspension. J. Environ. Manag. 2016, 171, 1–10. [Google Scholar] [CrossRef]
- Mohanty, K.; Naidu, J.T.; Meikap, B.C.; Biswas, M.N. Removal of Crystal Violet from Wastewater by Activated Carbons Prepared from Rice Husk. Ind. Eng. Chem. Res. 2006, 45, 5165–5171. [Google Scholar] [CrossRef]
- Au, W.; Pathak, S.; Collie, C.J.; Hsu, T.C. Cytogenetic toxicity of gentian violet and crystal violet on mammalian cells in vitro. Mutat. Res. Toxicol. 1978, 58, 269–276. [Google Scholar] [CrossRef]
- Rahimi, R.; Kerdari, H.; Rabbani, M. Adsorptive Removal of Crystal violet (CV), a Carcinogenic Textile Dye, from Aqueous Solution by Conducting Polyaniline/Hollow Manganese Ferrite Nanocomposites. In Proceedings of the ECSOC-14: The 14th International Electronic Conference on Synthetic Organic Chemistry, Basel, Switzerland, 1–30 November 2010. [Google Scholar]
- Bumajdad, A.; Eastoe, J.; Mathew, A. Cerium oxide nanoparticles prepared in self-assembled systems. Adv. Colloid Interface Sci. 2009, 147–148, 56–66. [Google Scholar] [CrossRef]
- Dubey, S.S.; Rao, B.S. Removal of cerium ions from aqueous solution by hydrous ferric oxide—A radiotracer study. J. Hazard. Mater. 2011, 186, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Hagen, A. Waste management-nuclear power, man and the environment. IAEA Bull. 2007, 24, 3–5. [Google Scholar]
- Hung, I.M.; Wang, H.P.; Lai, W.H.; Fung, K.Z.; Hon, M.H. Preparation of mesoporous cerium oxide templated by tri-block copolymer for solid oxide fuel cell. Electrochim. Acta 2004, 50, 745–748. [Google Scholar] [CrossRef]
- Ji, P.; Zhang, J.; Chen, F.; Anpo, M. Study of adsorption and degradation of acid orange 7 on the surface of CeO2 under visible light irradiation. Appl. Catal. B Environ. 2009, 85, 148–154. [Google Scholar] [CrossRef]
- Park, J.-W.; Jeong, J.-H.; Yoon, W.-L.; Jung, H.; Lee, H.-T.; Lee, D.-K.; Park, Y.-K.; Rhee, Y.-W. Activity and characterization of the Co-promoted CuO–CeO2/γ-Al2O3 catalyst for the selective oxidation of CO in excess hydrogen. Appl. Catal. Gen. 2004, 274, 25–32. [Google Scholar] [CrossRef]
- Yamashita, M.; Kameyama, K.; Yabe, S.; Yoshida, S.; Fujishiro, Y.; Kawai, T.; Sato, T. Synthesis and microstructure of calcia doped ceria as UV filters. J. Mater. Sci. 2002, 37, 683–687. [Google Scholar] [CrossRef]
- Filipi, R.; Nesměrák, K.; Rucki, M.; Roth, Z.; Hanzlíková, I.; Tichý, M. Acute toxicity of rare earth elements and their compounds. Chem. Listy 2007, 101, 793–798. [Google Scholar]
- Lin, W.; Huang, Y.-W.; Zhou, X.-D.; Ma, Y. Toxicity of Cerium Oxide Nanoparticles in Human Lung Cancer Cells. Int. J. Toxicol. 2006, 25, 451–457. [Google Scholar] [CrossRef]
- Rogers, N.J.; Franklin, N.M.; Apte, S.C.; Batley, G.E.; Angel, B.M.; Lead, J.R.; Baalousha, M. Physico-chemical behaviour and algal toxicity of nanoparticulate CeO2 in freshwater. Environ. Chem. 2010, 7, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Röhder, L.A.; Brandt, T.; Sigg, L.; Behra, R. Influence of agglomeration of cerium oxide nanoparticles and speciation of cerium(III) on short term effects to the green algae Chlamydomonas reinhardtii. Aquat. Toxicol. 2014, 152, 121–130. [Google Scholar] [CrossRef]
- Galle, P.; Berry, J.P.; Galle, C. Role of alveolar macrophages in precipitation of mineral elements inhaled as soluble aerosols. Environ. Health Perspect. 1992, 97, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.N.; Thomas, R.G.; McClellan, R.O. Influence of Chemical State of Cerium-144 on Its Metabolism Following Inhalation by Mice. Am. Ind. Hyg. Assoc. J. 1970, 31, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Talukder, G. Effects of metals on chromosomes of higher organisms. Environ. Mutagen. 1987, 9, 191–226. [Google Scholar] [CrossRef] [PubMed]
- Kartha, C.C.; Eapen, J.T.; Radhakumary, C.; Kutty, V.R.; Ramani, K.; Lal, A.V. Pattern of cardiac fibrosis in rabbits periodically fed a magnesium-restricted diet and administered rare earth chloride through drinking water. Biol. Trace Elem. Res. 1998, 63, 19–30. [Google Scholar] [CrossRef]
- Das, N.; Das, D. Recovery of rare earth metals through biosorption: An overview. J. Rare Earths 2013, 31, 933–943. [Google Scholar] [CrossRef]
- Klika, Z.; Seidlerová, J.; Valášková, M.; Kliková, C.; Kolomazník, I. Uptake of Ce(III) and Ce(IV) on montmorillonite. Appl. Clay Sci. 2016, 132–133, 41–49. [Google Scholar] [CrossRef]
- Erto, A.; Natale, F.; Musmarra, D.; Lancia, A. Modeling of single and competitive adsorption of cadmium and zinc onto activated carbon. Adsorption 2015, 21, 611–621. [Google Scholar] [CrossRef]
- Hand, D.W.; Loper, S.; Ari, M.; Crittenden, J.C. Prediction of multicomponent adsorption equilibria using ideal adsorbed solution theory. Environ. Sci. Technol. 1985, 19, 1037–1043. [Google Scholar] [CrossRef]
- Alekseeva, O.; Noskov, A.; Grishina, E.; Ramenskaya, L.; Kudryakova, N.; Ivanov, V.; Agafonov, A. Structural and Thermal Properties of Montmorillonite/Ionic Liquid Composites. Materials 2019, 12, 2578. [Google Scholar] [CrossRef] [Green Version]
- Channei, D.; Phanichphant, S.; Nakaruk, A.; Mofarah, S.; Koshy, P.; Sorrell, C. Aqueous and Surface Chemistries of Photocatalytic Fe-Doped CeO2 Nanoparticles. Catalysts 2017, 7, 45. [Google Scholar] [CrossRef]
- Nakada, R.; Takahashi, Y.; Tanimizu, M. Isotopic and speciation study on cerium during its solid-water distribution with implication for Ce stable isotope as a paleo-redox proxy. Geochim. Cosmochim. Acta 2013, 103, 49–62. [Google Scholar] [CrossRef]
- Nandi, B.K.; Goswami, A.; Das, A.K.; Mondal, B.; Purkait, M.K. Kinetic and Equilibrium Studies on the Adsorption of Crystal Violet Dye using Kaolin as an Adsorbent. Sep. Sci. Technol. 2008, 43, 1382–1403. [Google Scholar] [CrossRef]
- Asif Tahir, M.; Bhatti, H.N.; Iqbal, M. Solar Red and Brittle Blue direct dyes adsorption onto Eucalyptus angophoroides bark: Equilibrium, kinetics and thermodynamic studies. J. Environ. Chem. Eng. 2016, 4, 2431–2439. [Google Scholar] [CrossRef]
- Ullah, I.; Nadeem, R.; Iqbal, M.; Manzoor, Q. Biosorption of chromium onto native and immobilized sugarcane bagasse waste biomass. Ecol. Eng. 2013, 60, 99–107. [Google Scholar] [CrossRef]
- Merli, M.; Sciascia, L.; Turco Liveri, M.L. Regression diagnostics applied in kinetic data processing: Outlier recognition and robust weighting procedures. Int. J. Chem. Kinet. 2010, 42, 587–607. [Google Scholar] [CrossRef]
- Han, G.; Huang, Y.; Li, G.; Zhang, Y.; Jiang, T. Detailed Adsorption Studies of Active Humic Acid Fraction of a New Binder on Iron Ore Particles. Miner. Process. Extr. Metall. Rev. 2014, 35, 1–14. [Google Scholar] [CrossRef]
- Ma, Y.; Lyu, L.; Guo, Y.; Fu, Y.; Shao, Q.; Wu, T.; Guo, S.; Sun, K.; Guo, X.; Wujcik, E.; et al. Porous lignin based poly(acrylic acid)/organo-montmorillonite nanocomposites: Swelling behaviors and rapid removal of Pb(II) ions. Polymer 2017, 128, 12–23. [Google Scholar] [CrossRef]
- Sá, A.; Abreu, A.; Moura, I.; Vera Machado, A. Polymeric materials for metal sorption from hydric resources. In Water Purification; Academic Press, Elsevier: Cambridge, MA, USA, 2017; pp. 289–322. ISBN 978-0-12-804300-4. [Google Scholar]
- Sathishkumar, P.; Arulkumar, M.; Ashokkumar, V.; Mohd Yusoff, A.R.; Murugesan, K.; Palvannan, T.; Salam, Z.; Ani, F.N.; Hadibarata, T. Modified phyto-waste Terminalia catappa fruit shells: A reusable adsorbent for the removal of micropollutant diclofenac. RSC Adv. 2015, 5, 30950–30962. [Google Scholar] [CrossRef]
- Sakin Omer, O.; Hussein, M.A.; Hussein, B.H.M.; Mgaidi, A. Adsorption thermodynamics of cationic dyes (methylene blue and crystal violet) to a natural clay mineral from aqueous solution between 293.15 and 323.15 K. Arab. J. Chem. 2018, 11, 615–623. [Google Scholar] [CrossRef]
- Alhendawi, H.M.H.; Brunet, E.; Payán, E.R.; Juanes, O.; Ubis, J.C.R.; Al-Asqalany, M. Surfactant-assisted intercalation of crystal violet in layered γ-zirconium phosphate. Dye uptake from aqueous solutions. J. Incl. Phenom. Macrocycl. Chem. 2012, 73, 387–396. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, D.; Chang, Z.; Zhang, L. Adsorption of crystal violet onto amino silica: Optimization, equilibrium, and kinetic studies. Desalin. Water Treat. 2014, 52, 6113–6121. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Yu, K.; Ravi, S.; Ahn, W.-S. Selective Adsorption of Rare Earth Elements over Functionalized Cr-MIL-101. ACS Appl. Mater. Interfaces 2018, 10, 23918–23927. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.; Gao, Y.; Zhang, S.; Wu, K. Adsorption of Rare Earths(Ⅲ) Using an Efficient Sodium Alginate Hydrogel Cross-Linked with Poly-γ-Glutamate. PLoS ONE 2015, 10, e0124826. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, Y.; Wang, A. Preparation of granular hydrogel composite by the redox couple for efficient and fast adsorption of La(III) and Ce(III). J. Environ. Chem. Eng. 2015, 3, 1416–1425. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K. A Review on Heavy Metal Ions and Dye Adsorption from Water by Agricultural Solid Waste Adsorbents. Water Air Soil Pollut. 2018, 229, 225. [Google Scholar] [CrossRef]
- Chougui, A.; Zaiter, K.; Belouatek, A.; Asli, B. Heavy metals and color retention by a synthesized inorganic membrane. Arab. J. Chem. 2014, 7, 817–822. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.; He, Q.; Zhou, H.; Sun, X.; Zhang, J. Recovery of cerium(III) from aqueous solutions by complexation—ultrafiltration process. Asia Pac. J. Chem. Eng. 2012, 7, 940–947. [Google Scholar] [CrossRef]
- Grégorio, C.; Lichtfouse, É. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar]
- Dawodu, F.; Akpomie, K.; Abuh, M. Equilibrium Isotherm Studies on the Batch Sorption of Copper(II) ions from Aqueous Solution unto Nsu Clay. Int. J. Sci. Eng. Res. 2012, 3, 1–7. [Google Scholar]
- Malik, U.R.; Hasany, S.M.; Subhani, M.S. Sorptive potential of sunflower stem for Cr(III) ions from aqueous solutions and its kinetic and thermodynamic profile. Talanta 2005, 66, 166–173. [Google Scholar] [CrossRef]
- Singh, T.S.; Pant, K.K. Equilibrium, kinetics and thermodynamic studies for adsorption of As(III) on activated alumina. Sep. Purif. Technol. 2004, 36, 139–147. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Lazzara, G.; Merli, M.; Milioto, S.; Parisi, F.; Sciascia, L. Effect of the Biopolymer Charge and the Nanoclay Morphology on Nanocomposite Materials. Ind. Eng. Chem. Res. 2016, 55, 7373–7380. [Google Scholar] [CrossRef]
- Bromberg, L.; Straut, C.M.; Centrone, A.; Wilusz, E.; Hatton, T.A. Montmorillonite Functionalized with Pralidoxime As a Material for Chemical Protection against Organophosphorous Compounds. ACS Appl. Mater. Interfaces 2011, 3, 1479–1484. [Google Scholar] [CrossRef]
- Calabrese, I.; Cavallaro, G.; Lazzara, G.; Merli, M.; Sciascia, L.; Liveri, M.L.T. Preparation and characterization of bio-organoclays using nonionic surfactant. Adsorption 2016, 22, 105–116. [Google Scholar] [CrossRef]
- Cui, L.; Tarte, N.H.; Woo, S.I. Effects of Modified Clay on the Morphology and Properties of PMMA/Clay Nanocomposites Synthesized by in Situ Polymerization. Macromolecules 2008, 41, 4268–4274. [Google Scholar] [CrossRef]
- Mauro, N.; Chiellini, F.; Bartoli, C.; Gazzarri, M.; Laus, M.; Antonioli, D.; Griffiths, P.; Manfredi, A.; Ranucci, E.; Ferruti, P. RGD-mimic polyamidoamine–montmorillonite composites with tunable stiffness as scaffolds for bone tissue-engineering applications. J. Tissue Eng. Regen. Med. 2017, 11, 2164–2175. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, B.; Luo, J.; Ren, Y.; Zhang, Z. Efficient conversion of carbohydrates into 5-hydroxymethylfurfural catalyzed by the chromium-exchanged montmorillonite K-10 clay. Biomass Bioenergy 2014, 60, 171–177. [Google Scholar] [CrossRef]




| pH Conditions | ε, M−1 cm−1 | |
|---|---|---|
| CV (λ max = 591 nm) | pH 3.0 | 42000 ± 800 |
| pH 7.0 | 73800 ± 300 | |
| Ce(III) (λ max = 253 nm) | pH 3.0 | 740 ± 30 |
| pH 7.0 | 860 ± 50 |
| pH 3.0 | pH 5.0 | pH 7.0 | |
|---|---|---|---|
| CV | 73 wt% | 78 wt% | 95 wt% |
| Ce(III) | 0 | 0 | 50 wt% |
| Langmuir | ||||||
| qm, g g−1 | KL, dm3 g−1 | R2 | χ2 | ESS | ||
| CV | pH 3.0 | 0.155 ± 0.007 | 480 ± 60 | 0.969 | 6.0 × 10−5 | 1.3 × 10−3 |
| pH 7.0 | 0.22 ± 0.02 | 430 ± 70 | 0.961 | 8.8 × 10−5 | 1.0 × 10−3 | |
| Ce(III) | pH 7.0 | 0.122 ± 0.004 | 180 ± 20 | 0.984 | 1.0 × 10−5 | 9.2 × 10−5 |
| Freundlich | ||||||
| n | KF, (g g−1) (dm3 g−1)1/n | R2 | χ2 | ESS | ||
| CV | pH 3.0 | 2.2 ± 0.1 | 1.1 ± 0.1 | 0.985 | 2.9 × 10−5 | 6.3 × 10−4 |
| pH 7.0 | 2.0 ± 0.1 | 2.3 ± 0.4 | 0.967 | 7.4 × 10−5 | 8.8 × 10−4 | |
| Ce(III) | pH 7.0 | 2.5 ± 0.3 | 0.5 ± 0.1 | 0.923 | 5.0 × 10−5 | 4.5 × 10−4 |
| Xm, g g−1 | K, mol2 KJ−2 | E, KJ mol−1 | R2 | ||
|---|---|---|---|---|---|
| CV | pH = 3.0 | 0.47 ± 0.08 | (5.9 ± 0.2) × 10−4 | 9.2 ± 0.3 | 0.9599 |
| pH = 7.0 | 0.29 ± 0.05 | (6.7 ± 0.5) × 10−4 | 8.6 ± 0.6 | 0.9349 | |
| Ce(III) | pH = 7.0 | 0.23 ± 0.01 | (8.8 ± 0.7) × 10−4 | 7.5 ± 0.6 | 0.9377 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisi, F.; Lazzara, G.; Merli, M.; Milioto, S.; Princivalle, F.; Sciascia, L. Simultaneous Removal and Recovery of Metal Ions and Dyes from Wastewater through Montmorillonite Clay Mineral. Nanomaterials 2019, 9, 1699. https://doi.org/10.3390/nano9121699
Parisi F, Lazzara G, Merli M, Milioto S, Princivalle F, Sciascia L. Simultaneous Removal and Recovery of Metal Ions and Dyes from Wastewater through Montmorillonite Clay Mineral. Nanomaterials. 2019; 9(12):1699. https://doi.org/10.3390/nano9121699
Chicago/Turabian StyleParisi, Filippo, Giuseppe Lazzara, Marcello Merli, Stefana Milioto, Francesco Princivalle, and Luciana Sciascia. 2019. "Simultaneous Removal and Recovery of Metal Ions and Dyes from Wastewater through Montmorillonite Clay Mineral" Nanomaterials 9, no. 12: 1699. https://doi.org/10.3390/nano9121699
APA StyleParisi, F., Lazzara, G., Merli, M., Milioto, S., Princivalle, F., & Sciascia, L. (2019). Simultaneous Removal and Recovery of Metal Ions and Dyes from Wastewater through Montmorillonite Clay Mineral. Nanomaterials, 9(12), 1699. https://doi.org/10.3390/nano9121699