Fe3O4 Nanoparticles for Complex Targeted Delivery and Boron Neutron Capture Therapy
Abstract
:1. Introduction
2. Materials and Methods
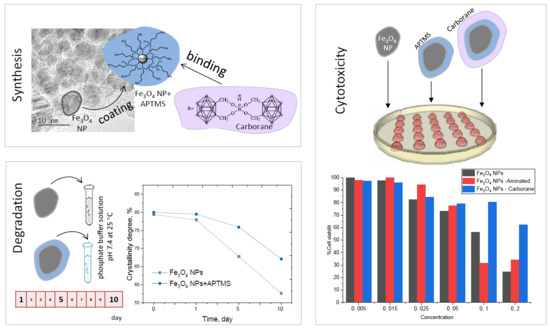
2.1. Synthesis and Modification of Fe3O4 NPs
2.2. Amine Functionalization of NPs and Carborane Immobilization
2.3. Methods of Characterization
2.4. Stability of Fe3O4 NPs
2.5. Cytotoxicity
2.5.1. Preparation of Cells
2.5.2. Cell Cytotoxicity Assay (MTT)
3. Results
3.1. Synthesis of Fe3O4 Nanoparticles
3.2. Modification of Fe3O4 Nanoparticles
- 1
- Dynamics of changes in structural properties over a model environment, similar in properties to biological fluids (PBS solution with an acidity of 7.4 t = 25 ° C).
- 2
- Immobilization of carboranes on the functionalized amino groups’ surface of the nanoparticles.
- 3
- Study of the toxicological properties of the magnetic carrier, magnetic carrier with a functionalized surface, and samples with immobilized carboranes.
3.3. Stability of Fe3O4 NPs in PBS Solution
3.4. Immobilization of Carborane Borate
3.5. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Remya, N.S.; Syama, S.; Sabareeswaran, A.; Mohanan, P.V. Toxicity, toxicokinetics and biodistribution of dextran stabilized Iron oxide Nanoparticles for biomedical applications. Int. J. Pharm. 2016, 511, 586–598. [Google Scholar] [CrossRef] [PubMed]
- Chandra Mohanta, S.; Saha, A.; Sujatha Devi, P. PEGylated Iron Oxide Nanoparticles for pH Responsive Drug Delivery Application. Mater. Today Proc. 2018, 5, 9715–9725. [Google Scholar] [CrossRef]
- Shelat, R.; Chandra, S.; Khanna, A. Detailed toxicity evaluation of β-cyclodextrin coated iron oxide nanoparticles for biomedical applications. Int. J. Biol. Macromol. 2018, 110, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.K.; Mitov, M.I.; Daley, E.F.; McGarry, R.C.; Anderson, K.W.; Hilt, J.Z. Targeted iron oxide nanoparticles for the enhancement of radiation therapy. Biomaterials 2016, 105, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Sun, W.; Xiao, Y.; Shi, X. Ultrasmall iron oxide nanoparticles: Synthesis, surface modification, assembly, and biomedical applications. Drug Discov. Today 2019, 24, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Khmara, I.; Strbak, O.; Zavisova, V.; Koneracka, M.; Kubovcikova, M.; Antal, I.; Kavecansky, V.; Lucanska, D.; Dobrota, D.; Kopcansky, P. Chitosan-stabilized iron oxide nanoparticles for magnetic resonance imaging. J. Magn. Magn. Mater. 2018, 474, 319–325. [Google Scholar] [CrossRef]
- Tay, Z.W.; Chandrasekharan, P.; Chiu-Lam, A.; Hensley, D.W.; Dhavalikar, R.; Zhou, X.Y.; Yu, E.Y.; Goodwill, P.W.; Zheng, B.; Rinaldi, C.; et al. Magnetic Particle Imaging-Guided Heating in Vivo Using Gradient Fields for Arbitrary Localization of Magnetic Hyperthermia Therapy. ACS Nano 2018, 12, 3699–3713. [Google Scholar] [CrossRef] [PubMed]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Mashhadizadeh, M.H.; Amoli-diva, M. Drug-Carrying Amino Silane Coated Magnetic Nanoparticles as Potential Vehicles for Delivery of Antibiotics. J. Nanomed. Nanotechnol. 2012, 3, 3–9. [Google Scholar] [CrossRef]
- Rajabi, F.; Arancon, R.A.D.; Luque, R. Oxidative esterification of alcohols and aldehydes using supported iron oxide nanoparticle catalysts. Catal. Commun. 2015, 59, 101–103. [Google Scholar] [CrossRef]
- Pizzolato, E.; Scaramuzza, S.; Carraro, F.; Sartori, A.; Agnoli, S.; Amendola, V.; Bonchio, M.; Sartorel, A. Water oxidation electrocatalysis with iron oxide nanoparticles prepared via laser ablation. J. Energy Chem. 2016, 25, 246–250. [Google Scholar] [CrossRef]
- Devi, H.S.; Singh, T.D. Iron oxide nanoparticles synthesis through a benign approach and its catalytic application. Perspect. Sci. 2016, 8, 287–289. [Google Scholar] [CrossRef] [Green Version]
- Nebu, J.; Anjali Devi, J.S.; Aparna, R.S.; Aswathy, B.; Lekha, G.M.; Sony, G. Fluorescence turn-on detection of fenitrothion using gold nanoparticle quenched fluorescein and its separation using superparamagnetic iron oxide nanoparticle. Sens. Actuators B Chem. 2018, 277, 271–280. [Google Scholar] [CrossRef]
- van de Loosdrecht, M.M.; Waanders, S.; Krooshoop, H.J.G.; ten Haken, B. Separation of excitation and detection coils for in vivo detection of superparamagnetic iron oxide nanoparticles. J. Magn. Magn. Mater. 2019, 475, 563–569. [Google Scholar] [CrossRef]
- Koda, J.; Venook, A.; Walser, E.; Goodwin, S. A multicenter, phase I/II trial of hepatic intra-arterial delivery of doxorubicin hydrochloride adsorbed to magnetic targeted carriers in patients with hepatocellular carcinoma. Eur. J. Cancer 2002, 38, S18. [Google Scholar]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhu, L.; Zhou, Y.; Chen, J. Accumulation and elimination of iron oxide nanomaterials in zebrafish (Danio rerio) upon chronic aqueous exposure. J. Environ. Sci. 2015, 30, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Stark, D.; Engelstad, B.; Bacon, B.; Compton, C.; White, D.; Jacobs, P.; Lewis, J. Superparamagnetic iron oxide: Pharmacokinetics and toxicity. Am. J. Roentgenol. 1989, 152, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Ozel, F.; Tokay, E.; Köçkar, F.; Köçkar, H. Characterization of tartaric acid and ascorbic acid coated iron oxide nanoparticles and their biocompatibility studies. J. Magn. Magn. Mater. 2018. [Google Scholar] [CrossRef]
- Yathindranath, V.; Sun, Z.; Worden, M.; Donald, L.J.; Thliveris, J.A. One-Pot Synthesis of Iron Oxide Nanoparticles with Functional Silane Shells: A Versatile General Precursor for Conjugations and Biomedical Applications. Langmuir 2013, 29, 10850–10858. [Google Scholar] [CrossRef]
- Özgür, M.; Ulu, A.; Balcıoğlu, S.; Özcan, İ.; Köytepe, S.; Ateş, B. The Toxicity Assessment of Iron Oxide (Fe3O4) Nanoparticles on Physical and Biochemical Quality of Rainbow Trout Spermatozoon. Toxics 2018, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bertólez, N.; Costa, C.; Brandão, F.; Kiliç, G.; Duarte, J.A.; Teixeira, J.P.; Pásaro, E.; Valdiglesias, V.; Laffon, B. Toxicological assessment of silica-coated iron oxide nanoparticles in human astrocytes. Food Chem. Toxicol. 2018, 118, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Janko, C.; Zaloga, J.; Pöttler, M.; Dürr, S.; Eberbeck, D.; Tietze, R.; Lyer, S.; Alexiou, C. Strategies to optimize the biocompatibility of iron oxide nanoparticles—“SPIONs safe by design”. J. Magn. Magn. Mater. 2017, 431, 281–284. [Google Scholar] [CrossRef]
- Alwi, R.; Telenkov, S.; Mandelis, A.; Leshuk, T.; Gu, F.; Oladepo, S.; Michaelian, K. Silica-coated super paramagnetic iron oxide nanoparticles (SPION) as biocompatible contrast agent in biomedical photoacoustics. Biomed. Opt. Express 2012, 3, 2500. [Google Scholar] [CrossRef] [PubMed]
- Kurczewska, J.; Cegłowski, M.; Schroeder, G. Preparation of multifunctional cascade iron oxide nanoparticles for drug delivery. Mater. Chem. Phys. 2018, 211, 34–41. [Google Scholar] [CrossRef]
- Nedunchezhian, K.; Aswath, N.; Thiruppathy, M.; Thirugnanamurthy, S. Boron Neutron Capture Therapy—A Literature Review. J. Clin. Diagn. Res. 2016, 10, ZE01–ZE04. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Matsumura, A. Feasibility for Intramedullary Spinal Glioma. In Neutron Capture Therapy; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2012; pp. 407–415. [Google Scholar]
- Aihara, T.; Morita, N.; Kamitani, N.; Kumada, H.; Ono, K.; Hiratsuka, J.; Harada, T. BNCT for advanced or recurrent head and neck cancer. Appl. Radiat. Isot. 2014, 88, 12–15. [Google Scholar] [CrossRef]
- Akan, Z. Boron Neutron Capture Therapy for Breast Cancer. Int. J. Women’s Heal. Reprod. Sci. 2015, 3, 77. [Google Scholar] [CrossRef]
- Henriksson, R.; Capala, J.; Michanek, A.; Lindahl, S.-Å.; Salford, L.G.; Franzén, L.; Blomquist, E.; Westlin, J.-E.; Bergenheim, A.T. Swedish Brain Tumour Study Group Boron neutron capture therapy (BNCT) for glioblastoma multiforme: A phase II study evaluating a prolonged high-dose of boronophenylalanine (BPA). Radiother. Oncol. 2008, 88, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Sauerwein, W.A.G. Principles and Roots of Neutron Capture Therapy. In Neutron Capture Therapy; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2012; pp. 1–16. [Google Scholar]
- Zakharkin, L.I.; Ol’shevskaya, V.A.; Spryshkova, R.A.; Grigor’eva, E.Y.; Ryabkova, V.I.; Borisov, G.I. Synthesis of bis(dialkylaminomethyl)-o- and m-carboranes and study of these compounds as potential preparations for boron neutron capture therapy. Pharm. Chem. J. 2000, 34, 301–304. [Google Scholar] [CrossRef]
- Barth, R.F.; Yang, W.; Soloway, A.H. Strategies to Improve the Efficacy of Neutron Capture Therapy of Brain Tumors by Optimizing Delivery of Boron Compounds. In Frontiers in Neutron Capture Therapy; Springer US: Boston, MA, USA, 2001; pp. 1093–1101. [Google Scholar]
- Sumitani, S.; Oishi, M.; Nagasaki, Y. Carborane confined nanoparticles for boron neutron capture therapy: Improved stability, blood circulation time and tumor accumulation. React. Funct. Polym. 2011, 71, 684–693. [Google Scholar] [CrossRef] [Green Version]
- Pak, R.H.; Primus, F.J.; Rickard-Dickson, K.J.; Ng, L.L.; Kane, R.R.; Hawthorne, M.F. Preparation and properties of nido-carborane-specific monoclonal antibodies for potential use in boron neutron capture therapy for cancer. Proc. Natl. Acad. Sci. USA 1995, 92, 6986–6990. [Google Scholar] [CrossRef]
- Miyajima, Y.; Nakamura, H.; Kuwata, Y.; Lee, J.-D.; Masunaga, S.; Ono, K. Kazuo Maruyama Transferrin-Loaded nido-Carborane Liposomes: Tumor-Targeting Boron Delivery System for Neutron Capture Therapy. Bioconjug. Chem. 2006, 17, 1314–1320. [Google Scholar] [CrossRef]
- Altieri, S.; Balzi, M.; Bortolussi, S.; Bruschi, P.; Ciani, L.; Clerici, A.M.; Faraoni, P.; Ferrari, C.; Gadan, M.A.; Panza, L.; et al. Carborane Derivatives Loaded into Liposomes as Efficient Delivery Systems for Boron Neutron Capture Therapy. J. Med. Chem. 2009, 52, 7829–7835. [Google Scholar] [CrossRef] [PubMed]
- Yinghuai, Z.; Peng, A.T.; Carpenter, K.; Maguire, J.A.; Hosmane, N.S. Masao Takagaki Substituted Carborane-Appended Water-Soluble Single-Wall Carbon Nanotubes: New Approach to Boron Neutron Capture Therapy Drug Delivery. J. Am. Chem. Soc. 2005, 127, 9875–9880. [Google Scholar] [CrossRef]
- Cioran, A.M.; Teixidor, F.; Krpetić, Ž.; Brust, M.; Viñas, C. Preparation and characterization of Au nanoparticles capped with mercaptocarboranyl clusters. Dalton Trans. 2014, 43, 5054–5061. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lin, Y.; Zhu, Y.Z.; Lu, J.; Maguire, J.A.; Hosmane, N.S. Boron Drug Delivery via Encapsulated Magnetic Nanocomposites: A New Approach for BNCT in Cancer Treatment. J. Nanomater. 2010, 2010, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, A.; Hosmane, N.S. Nanotechnology-driven chemistry of boron materials. Pure Appl. Chem. 2012, 84, 2299–2308. [Google Scholar] [CrossRef]
- Bietenbeck, M.; Florian, A.; Faber, C.; Sechtem, U.; Yilmaz, A. Remote magnetic targeting of iron oxide nanoparticles for cardiovascular diagnosis and therapeutic drug delivery: Where are we now? Int. J. Nanomed. 2016, 11, 3191–3203. [Google Scholar]
- Korolkov, I.V.; Kozlovskiy, A.L.; Gorin, Y.G.; Kazantsev, A.V.; Shlimas, D.I.; Zdorovets, M.V.; Ualieva, N.K.; Rusakov, V.S. Immobilization of carborane derivatives on Ni/Fe nanotubes for BNCT. J. Nanopart. Res. 2018, 20, 240. [Google Scholar] [CrossRef]
- Freshney, R.I.; Wiley InterScience (Online service). Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications; Wiley-Blackwell: Oxford, UK, 2010; ISBN 9780470649367. [Google Scholar]
- Bayreuther, K.; Francz, P.I.; Gogol, J.; Hapke, C.; Maier, M.; Meinrath, H.G. Differentiation of primary and secondary fibroblasts in cell culture systems. Mutat. Res. 1991, 256, 233–242. [Google Scholar] [CrossRef]
- Onar, K.; Yakinci, M.E. Synthesis of Fe3O4nanoparticles for biomedical applications. J. Phys. Conf. Ser. 2016, 667, 5–10. [Google Scholar] [CrossRef]
- Si, S.; Kotal, A.; Mandal, T.K.; Giri, S.; Hiroyuki Nakamura, A.; Kohara, T. Size-Controlled Synthesis of Magnetite Nanoparticles in the Presence of Polyelectrolytes. Chem. Mater. 2004, 16, 3489–3496. [Google Scholar] [CrossRef]
- Daou, T.J.; Pourroy, G.; Bégin-Colin, S.; Grenèche, J.M.; Ulhaq-Bouillet, C.; Legaré, P.; Bernhardt, P.; Leuvrey, C.; Rogez, G. Hydrothermal Synthesis of Monodisperse Magnetite Nanoparticles. Chem. Mater. 2006, 18, 4399–4404. [Google Scholar] [CrossRef]
- Ghosh, R.; Pradhan, L.; Devi, Y.P.; Meena, S.S.; Tewari, R.; Kumar, A.; Sharma, S.; Gajbhiye, N.S.; Vatsa, R.K.; Pandey, B.N.; et al. Induction heating studies of Fe3O4 magnetic nanoparticles capped with oleic acid and polyethylene glycol for hyperthermia. J. Mater. Chem. 2011, 21, 13388. [Google Scholar] [CrossRef]
- Yurenya, A.; Nikitin, A.; Garanina, A.; Gabbasov, R.; Polikarpov, M.; Cherepanov, V.; Chuev, M.; Majouga, A.; Panchenko, V. Synthesis and Mössbauer study of 57Fe-based nanoparticles biodegradation in living cells. J. Magn. Magn. Mater. 2019, 474, 337–342. [Google Scholar] [CrossRef]
- Lyubutin, I.S.; Lin, C.R.; Korzhetskiy, Y.V.; Dmitrieva, T.V.; Chiang, R.K. Mössbauer spectroscopy and magnetic properties of hematite/magnetite nanocomposites. J. Appl. Phys. 2009, 106, 034311. [Google Scholar] [CrossRef]
- Kandasamy, G.; Maity, D. Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int. J. Pharm. 2015, 496, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, M.; Ahmad, M.; Haron, M.; Namvar, F.; Nadi, B.; Rahman, M.; Amin, J. Synthesis, Surface Modification and Characterisation of Biocompatible Magnetic Iron Oxide Nanoparticles for Biomedical Applications. Molecules 2013, 18, 7533–7548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Wang, S.; Wen, M.; Shen, R.; Guo, X.; Cao, J.; Wang, X. Aminopropyltriethoxysilane-mediated surface functionalization of hydroxyapatite nanoparticles: Synthesis, characterization, and in vitro toxicity assay. Int. J. Nanomed. 2011, 6, 3449. [Google Scholar] [CrossRef]
- Sawatzky, G.A.; Coey, J.M.D.; Morrish, A.H. Mössbauer Study of Electron Hopping in the Octahedral Sites of Fe3O4. J. Appl. Phys. 1969, 40, 1402–1403. [Google Scholar] [CrossRef]
- Iyengar, S.J.; Joy, M.; Ghosh, C.K.; Dey, S.; Kotnala, R.K.; Ghosh, S. Magnetic, X-ray and Mössbauer studies on magnetite/maghemite core–shell nanostructures fabricated through an aqueous route. RSC Adv. 2014, 4, 64919–64929. [Google Scholar] [CrossRef] [Green Version]
- Arsalani, N.; Fattahi, H.; Nazarpoor, M. Synthesis and characterization of PVP-functionalized superparamagnetic Fe3O4 nanoparticles as an MRI contrast agent. Express Polym. Lett. 2010, 4, 329–338. [Google Scholar] [CrossRef]
- Chowdhuri, A.R.; Bhattacharya, D.; Sahu, S.K. Magnetic nanoscale metal organic frameworks for potential targeted anticancer drug delivery, imaging and as an MRI contrast agent. Dalt. Trans. 2016, 45, 2963–2973. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J. Magnetic nanoparticles for drug delivery. Drug Dev. Res. 2006, 67, 55–60. [Google Scholar] [CrossRef]
- Tolouei, R.; Harrison, J.; Paternoster, C.; Turgeon, S.; Chevallier, P.; Mantovani, D. The use of multiple pseudo-physiological solutions to simulate the degradation behavior of pure iron as a metallic resorbable implant: A surface-characterization study. Phys. Chem. Chem. Phys. 2016, 18, 19637–19646. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.; Marciello, M.; del Puerto Morales, M.; Serna, C.; Vargas, M.; Ronconi, C.; Costo, R. Studies of the Colloidal Properties of Superparamagnetic Iron Oxide Nanoparticles Functionalized with Platinum Complexes in Aqueous and PBS Buffer Media. J. Braz. Chem. Soc. 2016, 28, 731–739. [Google Scholar] [CrossRef]
- Li, X.; Lachmanski, L.; Safi, S.; Sene, S.; Serre, C.; Grenèche, J.M.; Zhang, J.; Gref, R. New insights into the degradation mechanism of metal-organic frameworks drug carriers. Sci. Rep. 2017, 7, 13142. [Google Scholar] [CrossRef] [Green Version]
- Kozlovskiy, A.; Dukenbayev, K.; Ivanov, I.; Kozin, S.; Aleksandrenko, V.; Kurakhmedov, A.; Sambaev, E.; Kenzhina, I.; Tosi, D.; Loginov, V.; et al. Investigation of the influence of irradiation with Fe+7 ions on structural properties of AlN ceramics. Mater. Res. Express 2018, 5, 065502. [Google Scholar] [CrossRef]
- Mendelovici, E.; Yariv, S. The Effect of Degree of Crystallinity on the Infrared Spectrum of Hematite. Appl. Spectrosc. 1979, 33, 410–411. [Google Scholar]
- Tan, C.; Gao, N.; Deng, Y.; Deng, J.; Zhou, S.; Li, J.; Xin, X. Radical induced degradation of acetaminophen with Fe3O4 magnetic nanoparticles as heterogeneous activator of peroxymonosulfate. J. Hazard. Mater. 2014, 276, 452–460. [Google Scholar] [CrossRef]
- Deng, J.; Shao, Y.; Gao, N.; Tan, C.; Zhou, S.; Hu, X. CoFe2O4 magnetic nanoparticles as a highly active heterogeneous catalyst of oxone for the degradation of diclofenac in water. J. Hazard. Mater. 2013, 262, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Spanhel, L.; Anderson, M.A. Semiconductor clusters in the sol-gel process: Quantized aggregation, gelation, and crystal growth in concentrated zinc oxide colloids. J. Am. Chem. Soc. 1991, 113, 2826–2833. [Google Scholar] [CrossRef]
- Ye, F.; Barrefelt, Å.; Asem, H.; Abedi-Valugerdi, M.; El-Serafi, I.; Saghafian, M.; Abu-Salah, K.; Alrokayan, S.; Muhammed, M.; Hassan, M. Biodegradable polymeric vesicles containing magnetic nanoparticles, quantum dots and anticancer drugs for drug delivery and imaging. Biomaterials 2014, 35, 3885–3894. [Google Scholar] [CrossRef] [PubMed]
- Chorny, M.; Hood, E.; Levy, R.J.; Muzykantov, V.R. Endothelial delivery of antioxidant enzymes loaded into non-polymeric magnetic nanoparticles. J. Control. Release 2010, 146, 144–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabbasov, R.; Cherepanov, V.; Chuev, M.; Polikarpov, M.; Nikitin, M.; Deyev, S.; Panchenko, V. Biodegradation of Magnetic Nanoparticles in Mouse Liver From Combined Analysis of Mössbauer and Magnetization Data. IEEE Trans. Magn. 2013, 49, 394–397. [Google Scholar] [CrossRef]
- Nikitin, M.; Gabbasov, R.; Cherepanov, V.; Chuev, M.; Polikarpov, M.; Panchenko, V.; Deyev, S. Magnetic Nanoparticle Degradation in vivo Studied by Mössbauer Spectroscopy. AIP Conf. Proc. 2010, 1311, 401–407. [Google Scholar]
- Buxbaum, L.H. The Degradation of Poly (ethy1ene terephthalate). Angew. Chem. Internat. Ed. 1968, 7, 182–190. [Google Scholar] [CrossRef]
- Pasternack, R.M.; Amy, S.R.; Chabal, Y.J. Attachment of 3-(Aminopropyl) triethoxysilane on Silicon Oxide Surfaces: Dependence on Solution Temperature. Langmuir 2008, 7, 12963–12971. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.K.; Reddy, M.K.; Morales, M.A.; Leslie-Pelecky, D.L.; Labhasetwar, V. Biodistribution, Clearance, and Biocompatibility of Iron Oxide Magnetic Nanoparticles in Rats. Mol. Pharm. 2008, 5, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2010, 110, 2574. [Google Scholar] [CrossRef]
- Hong, S.; Bielinska, A.U.; Mecke, A.; Keszler, B.; Beals, J.L.; Shi, X.; Balogh, L.; Orr, B.G.; Baker, J.R.; Banaszak Holl, M.M. Interaction of Poly(amidoamine) Dendrimers with Supported Lipid Bilayers and Cells: Hole Formation and the Relation to Transport. Bioconjug. Chem. 2004, 15, 774–782. [Google Scholar] [CrossRef] [PubMed]







| Sample | Atomic Content, % | [NH2], µM/g | |||
|---|---|---|---|---|---|
| N | Fe | Si | O | ||
| Fe3O4 NPs | - | 43.1 | - | 56.9 | - |
| Fe3O4-Aminated NPs | 1.0 | 36.0 | 2.3 | 60.7 | 86.57 |
| Analysis | Parameter | Fe3O4 | Fe3O4-Aminated NPs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Degradation, days | 0 | 1 | 5 | 10 | 0 | 1 | 5 | 10 | ||
| XRD | Relative change of lattice parameter, % (a0 = 8.34800 Å, PDF—01-075-9673) | 0.074 | 0.089 | 0.097 | 0.103 | 0.021 | 0.035 | 0.047 | 0.048 | |
| Crystalline size, nm | 10.3 ± 0.7 | 12.1 ± 0.6 | 12.2 ± 0.9 | 12.9 ± 0.3 | 12.8 ± 0.8 | 12.5 ± 0.7 | 12.7 ± 0.4 | 12.8 ± 1.1 | ||
| Crystallinity, % | 79.4 ± 3.1 | 77.9 ± 1.5 | 67.8 ± 2.4 | 57.6 ± 2.3 | 80.1 ± 1.8 | 79.5 ± 1.5 | 75.9 ± 1.4 | 67.1 ± 2.2 | ||
| DLS | Average size of particles, nm | 18.9 ± 3.2 | 19.66 ± 3.14 | 26.52 ± 4.1 | 27.88 ± 3.9 | 21.8 ± 3.56 | 21.4 ± 3.7 | 23.46 ± 4.23 | 26.22 ± 4.15 | |
| Mossbauer | Hyperfine field, kOe | A-site | 472.9 ± 2.5 | 453.2 ± 3.5 | 434.1 ± 4.6 | 421.6 ± 2.7 | 466.8 ± 8.6 | 452.2 ± 4.3 | 441.3 ± 4.7 | 422.2 ± 4.3 |
| B-site | 442 ± 4.1 | 434 ± 3.2 | 432 ± 4.4 | 417 ± 2.2 | 432 ± 1.7 | 426 ± 2.2 | 421 ± 2.4 | 418 ± 2.3 | ||
| Sample | Atomic Content, % | ||||
|---|---|---|---|---|---|
| B | N | Fe | Si | O | |
| Fe3O4-Carborane NPs | 11.4 | 2.0 | 33.5 | 12.2 | 40.9 |
| No. | Sample | IC50, mg/mL |
|---|---|---|
| 1 | Fe3O4 NPs | 0.110 |
| 2 | Fe3O4-Aminated NPs | 0.091 |
| 3 | Fe3O4-Carboranes NPs | 0.405 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dukenbayev, K.; Korolkov, I.V.; Tishkevich, D.I.; Kozlovskiy, A.L.; Trukhanov, S.V.; Gorin, Y.G.; Shumskaya, E.E.; Kaniukov, E.Y.; Vinnik, D.A.; Zdorovets, M.V.; et al. Fe3O4 Nanoparticles for Complex Targeted Delivery and Boron Neutron Capture Therapy. Nanomaterials 2019, 9, 494. https://doi.org/10.3390/nano9040494
Dukenbayev K, Korolkov IV, Tishkevich DI, Kozlovskiy AL, Trukhanov SV, Gorin YG, Shumskaya EE, Kaniukov EY, Vinnik DA, Zdorovets MV, et al. Fe3O4 Nanoparticles for Complex Targeted Delivery and Boron Neutron Capture Therapy. Nanomaterials. 2019; 9(4):494. https://doi.org/10.3390/nano9040494
Chicago/Turabian StyleDukenbayev, Kanat, Ilya V. Korolkov, Daria I. Tishkevich, Artem L. Kozlovskiy, Sergey V. Trukhanov, Yevgeniy G. Gorin, Elena E. Shumskaya, Egor Y. Kaniukov, Denis A. Vinnik, Maxim V. Zdorovets, and et al. 2019. "Fe3O4 Nanoparticles for Complex Targeted Delivery and Boron Neutron Capture Therapy" Nanomaterials 9, no. 4: 494. https://doi.org/10.3390/nano9040494
APA StyleDukenbayev, K., Korolkov, I. V., Tishkevich, D. I., Kozlovskiy, A. L., Trukhanov, S. V., Gorin, Y. G., Shumskaya, E. E., Kaniukov, E. Y., Vinnik, D. A., Zdorovets, M. V., Anisovich, M., Trukhanov, A. V., Tosi, D., & Molardi, C. (2019). Fe3O4 Nanoparticles for Complex Targeted Delivery and Boron Neutron Capture Therapy. Nanomaterials, 9(4), 494. https://doi.org/10.3390/nano9040494