A Cross-Linker-Based Poly(Ionic Liquid) for Sensitive Electrochemical Detection of 4-Nonylphenol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
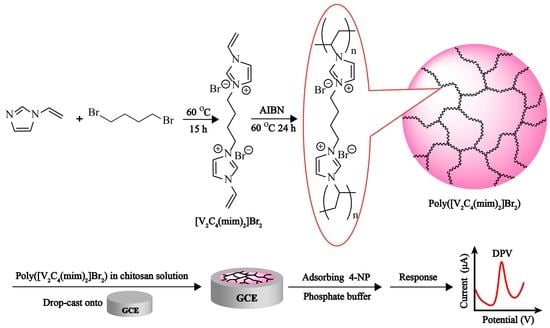
2.3. Preparation of Poly(Ionic Liquid) with [V2C4(mim)2]Br2 as Cross-Linker
2.4. Electrochemical Measurements
3. Results
3.1. Characterization of Poly([V2C4(mim)2]Br2)
3.2. Electrochemical Behavior of Poly([V2C4(mim)2]Br2)
3.3. Optimization of Experimental Conditions
3.4. Analytical Performance of Poly([V2C4(mim)2]Br2)/GCE
3.5. Reproducibility, Stability and Selectivity of Poly([V2C4(mim)2]Br2)/GCE
3.6. Analytical Application
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lu, Q.; Zhang, W.; Wang, Z.; Yu, G.; Yuan, Y.; Zhou, Y. A facile electrochemical sensor for nonylphenol determination based on the enhancement effect of cetyltrimethylammonium bromide. Sensors 2013, 13, 758–768. [Google Scholar] [CrossRef]
- Soares, A.; Guieysse, B.; Jefferson, B.; Cartmell, E.; Lester, J. Nonylphenol in the environment: A critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ. Int. 2008, 34, 1033–1049. [Google Scholar] [CrossRef]
- Preuss, T.G.; Gehrhardt, J.; Schirmer, K.; Coors, A.; Rubach, M.; Russ, A.; Jones, P.D.; Giesy, J.P.; Ratte, H.T. Nonylphenol isomers differ in estrogenic activity. Environ. Sci. Technol. 2006, 40, 5147–5153. [Google Scholar] [CrossRef] [PubMed]
- Céspedes, R.; Skryjová, K.; Raková, M.; Zeravik, J.; Fránek, M.; Lacorte, S.; Barceló, D. Validation of an enzyme-linked immunosorbent assay (ELISA) for the determination of 4-nonylphenol and octylphenol in surface water samples by LC-ESI-MS. Talanta 2006, 70, 745–751. [Google Scholar] [CrossRef]
- Lopes, D.; Dias, A.N.; Merib, J.; Carasek, E. Hollow-fiber renewal liquid membrane extraction coupled with 96-well plate system as innovative high-throughput configuration for the determination of endocrine disrupting compounds by high-performance liquid chromatography-fluorescence and diode array detection. Anal. Chim. Acta 2018, 1040, 33–40. [Google Scholar]
- Pastor-Belda, M.; Viñas, P.; Campillo, N.; Hernández-Córdoba, M. Magnetic solid phase extraction with CoFe2O4/oleic acid nanoparticles coupled to gas chromatography-mass spectrometry for the determination of alkylphenols in baby foods. Food. Chem. 2017, 221, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Li, X.; Wang, Y.; Su, C. A core–shell Fe3O4 nanoparticle–CdTe quantum dot–molecularly imprinted polymer composite for recognition and separation of 4-nonylphenol. Anal. Methods 2014, 6, 2855–2861. [Google Scholar] [CrossRef]
- Ai, J.; Guo, H.; Xue, R.; Wang, X.; Lei, X.; Yang, W. A self-probing, gate-controlled, molecularly imprinted electrochemical sensor for ultrasensitive determination of p-nonylphenol. Electrochem. Commun. 2018, 89, 1–5. [Google Scholar] [CrossRef]
- Qin, J.; Guo, J.; Xu, Q.; Zheng, Z.; Mao, H.; Yan, F. Synthesis of pyrrolidinium-type poly(ionic liquid) membranes for antibacterial applications. ACS Appl. Mater. Int. 2017, 9, 10504–10511. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Liu, R.; Zhang, J.; Qi, X.; He, Y.; Li, B.; Yang, Q.; Hu, Y.; Kang, F. In situ synthesis of hierarchical poly(ionic liquid)-based solid electrolytes for high-safety lithium-ion and sodium-ion batteries. Nano Energy 2017, 33, 45–54. [Google Scholar] [CrossRef]
- Osada, I.; de Vries, H.; Scrosati, B.; Passerini, S. Ionic-Liquid-Based Polymer Electrolytes for Battery Applications. Angew. Chem. Int. Ed. 2016, 55, 500–513. [Google Scholar] [CrossRef]
- Qian, W.; Texter, J.; Yan, F. Frontiers in poly(ionic liquid)s: Syntheses and applications. Chem. Soc. Rev. 2017, 46, 1124–1159. [Google Scholar] [CrossRef]
- Wang, P.; Wang, T.; Lin, W.; Lin, H.; Lee, M.; Yang, C. Crosslinked polymer ionic liquid/ionic liquid blends prepared by photopolymerization as solid-state electrolytes in supercapacitors. Nanomaterials 2018, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.J.; Chen, G.J.; Wang, X.C.; Li, J.; Zhou, Y.; Wang, J. A hierarchical meso-macroporous poly(ionic liquid) monolith derived from a single soft template. Chem. Commun. 2015, 51, 4969–4972. [Google Scholar] [CrossRef]
- Suo, X.; Xia, L.; Yang, Q.; Zhang, Z.; Bao, Z.; Ren, Q.; Yang, Y.; Xing, H. Synthesis of anion-functionalized mesoporous poly(ionic liquid)s via a microphase separation-hypercrosslinking strategy: Highly efficient adsorbents for bioactive molecules. J. Mater. Chem. A 2017, 5, 14114–14123. [Google Scholar] [CrossRef]
- Wu, M.; Wang, L.; Zeng, B.; Zhao, F. Ionic liquid polymer functionalized carbon nanotubes-doped poly(3,4-ethylenedioxythiophene) for highly-efficient solid-phase microextraction of carbamate pesticides. J. Chromatogr. A 2016, 1444, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yuan, Y.; Yang, X.; Han, Y.; Yan, H. Polymethacrylate microparticles covalently functionalized with an ionic liquid for solid-phase extraction of fluoroquinolone antibiotics. Microchim. Acta 2015, 182, 2201–2208. [Google Scholar] [CrossRef]
- Chen, L.; Mei, M.; Huang, X.; Yuan, D. Sensitive determination of estrogens in environmental waters treated with polymeric ionic liquid-based stir cake sorptive extraction and liquid chromatographic analysis. Talanta 2016, 152, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liao, Y.; Huang, X. Fabrication of polymeric ionic liquid-modified magnetic adsorbent for extraction of apolar and polar pollutants in complicated samples. Talanta 2017, 172, 23–30. [Google Scholar] [CrossRef]
- Liu, C.; Deng, Q.; Fang, G.; Dang, M.; Wang, S. Capillary electrochromatography immunoassay for alpha-fetoprotein based on poly(guanidinium ionic liquid) monolithic material. Anal. Biochem. 2017, 530, 50–56. [Google Scholar] [CrossRef]
- Wang, Q.; Yun, Y. Nonenzymatic sensor for hydrogen peroxide based on the electrodeposition of silver nanoparticles on poly(ionic liquid)-stabilized graphene sheets. Microchim. Acta 2013, 180, 261–268. [Google Scholar] [CrossRef]
- Sánchez-Paniagua López, M.; López-Ruiz, B. Electrochemical biosensor based on ionic liquid polymeric microparticles. An analytical platform for catechol. Microchem. J. 2018, 138, 173–179. [Google Scholar] [CrossRef]
- Cui, K.; Lu, X.; Cui, W.; Wu, J.; Chen, X.; Lu, Q. Fluorescent nanoparticles assembled from a poly(ionic liquid) for selective sensing of copper ions. Chem. Commun. 2011, 47, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Wu, T.; Ye, X. Polymerized ionic liquid functionalized graphene oxide nanosheets as a sensitive platform for bisphenol A sensing. Carbon 2018, 129, 21–28. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, C.; Yin, T.; Ou, S.; Sun, X.; Wen, X.; Zhang, L.; Tang, D.; Yin, X. Functionalized poly (ionic liquid) as the support to construct a ratiometric electrochemical biosensor for the selective determination of copper ions in AD rats. Biosens. Bioelectron. 2017, 87, 278–284. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Zeng, Y.; Tang, T.; Pan, Y.; Li, L. An electrochemical sensor for the sensitive determination of phenylethanolamine A based on a novel composite of reduced graphene oxide and poly (ionic liquid). RSC Adv. 2015, 5, 717–725. [Google Scholar] [CrossRef]
- Ren, J.; Gu, J.; Tao, L.; Yao, M.; Yang, X.; Yang, W. A novel electrochemical sensor of 4-nonylphenol based on a poly(ionic liquid) hollow nanosphere/gold nanoparticle composite modified glassy carbon electrode. Anal. Methods 2015, 7, 8094–8099. [Google Scholar] [CrossRef]
- Ding, S.; Hu, X.; Guan, P.; Zhang, N.; Li, J.; Gao, X.; Zhang, X.; Ding, X.; Du, C. Preparation of surface-imprinted microspheres using ionic liquids as novel cross-linker for recognizing an immunostimulating peptide. J. Mater. Sci. 2017, 52, 8027–8040. [Google Scholar] [CrossRef]
- Ma, W.W.; Row, K.H. Solid-phase extraction of chlorophenols in seawater using a magnetic ionic liquid molecularly imprinted polymer with incorporated silicon dioxide as a sorbent. J. Chromatogr. A 2018, 1559, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zeng, Y.; Zhang, Z.; Yang, Y.; Zhai, Y.; Wang, H.; Liu, L.; Hu, J.; Li, L. A new composite of graphene and molecularly imprinted polymer based on ionic liquids as functional monomer and cross-linker for electrochemical sensing 6-benzylaminopurine. Biosens. Bioelectron. 2018, 108, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Antonietti, M. Poly (ionic liquid) latexes prepared by dispersion polymerization of ionic liquid monomers. Macromolecules 2011, 44, 744–750. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, S.; Zhang, L.; Lu, J.; Verproot, F.; Liu, Y.; Xing, Z.; Li, J.; Song, X. Fabrication of polymeric ionic liquid/graphene nanocomposite for glucose oxidase immobilization and direct electrochemistry. Biosens. Bioelectron. 2011, 26, 2632–2637. [Google Scholar] [CrossRef]
- Mao, Y.; Bao, Y.; Gan, S.; Li, F.; Niu, L. Electrochemical sensor for dopamine based on a novel graphene-molecular imprinted polymers composite recognition element. Biosens. Bioelectron. 2011, 28, 291–297. [Google Scholar] [CrossRef]
- Ana-Maria, G.; Lucian, R.; Mihaela, B.; Ioan, B.; Camelia, B. Sensitive detection of endocrine disrupters using ionic liquid--single walled carbon nanotubes modified screen-printed based biosensors. Talanta 2011, 85, 2007–2013. [Google Scholar]
- Filipecka, K.; MiedzińSki, R.; Sitarz, M.; Filipecki, J.; Makowska-Janusik, M. Optical and vibrational properties of phosphorylcholine-based contact lenses-experimental and theoretical investigations. Spectrochim. Acta A 2017, 176, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Anson, F.C. Application of Potentiostatic Current Integration to the Study of the Adsorption of Cobalt(III)-(Ethylenedinitrilo(tetraacetate) on Mercury Electrodes. Anal. Chem. 1964, 36, 932–934. [Google Scholar] [CrossRef]
- Li, S.; Lei, S.; Yu, Q.; Zou, L.; Ye, B. A novel electrochemical sensor for detecting hyperin with a nanocomposite of ZrO2-SDS-SWCNTs as decoration. Talanta 2018, 185, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Zhang, Y.; Wang, Z.; Wan, Q.; Yang, N. Decoration of graphene nano platelets with gold nanoparticles for voltammetry of 4-nonylphenol. Carbon 2017, 117, 313–321. [Google Scholar] [CrossRef]
- Xue, F.; Gao, Z.; Sun, X.; Yang, Z.; Yi, L.; Chen, W. Electrochemical determination of environmental hormone nonylphenol based on composite film modified gold electrode. J. Electrochem. Soc. 2015, 162, H338–H344. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, A.; Zhu, X.; Zhang, C.; Liang, Y.; Nan, J. Electrochemical determination of nonylphenol using differential pulse voltammetry based on a graphene–DNA-modified glassy carbon electrode. J. Electroanal. Chem. 2013, 703, 153–157. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, P.; Wan, Q.; Yang, N. Integration of chromium terephthalate metal-organic frameworks with reduced graphene oxide for voltammetry of 4-nonylphenol. Carbon 2018, 134, 540–547. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, X.; Liu, S.; Lin, Q.; He, X.; Xing, X.; Lian, W.; Tang, D. Development of molecularly imprinted electrochemical sensor with titanium oxide and gold nanomaterials enhanced technique for determination of 4-nonylphenol. Sens. Actuators B-Chem. 2011, 152, 292–298. [Google Scholar] [CrossRef]
- Meng, X.; Yin, H.; Xu, M.; Ai, S.; Zhu, J. Electrochemical determination of nonylphenol based on ionic liquid-functionalized graphene nanosheet modified glassy carbon electrode and its interaction with DNA. J. Solid State Electr. 2012, 16, 2837–2843. [Google Scholar] [CrossRef]








| Detection Method | Linear Range (μM) | Detection Limit (μM) | Ref. |
|---|---|---|---|
| CTAB a/carbon paste electrode | 0.1–25 | 0.01 | [1] |
| AuNPs/PILs b/GCE | 0.1–120 | 0.033 | [27] |
| β-CD-SH-GR c/Au electrode | 0.07–70 | 0.061 | [39] |
| GR d-DNA/GCE | 0.05–4 | 0.01 | [40] |
| MIL-101(Cr)@Rgo e/GCE | 0.1–12.5 | 0.033 | [41] |
| MIP f/AuNPs/TiO2/GCE | 0.95–480 | 0.32 | [42] |
| IL-FGNS g/GCE | 0.5–200 | 0.058 | [43] |
| Poly([V2C4(mim)2]Br2)/GCE | 0.05–5 | 0.01 | This work |
| Foreign Species | Tolerable Ratio |
|---|---|
| Na+, K+, Fe2+, Mg2+, Ni2+, Co2+, Cu2+, Cl−, NO3−, SO42− | 100 |
| Hydroquinone, catechol, benzene, 1,2-dimethylbenzene, 2-nitroaniline, 3-nitroanilie, 4-nitroaniline | 10 |
| Sample | Added 4-NP (μM) | Found 4-NP (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Lake water | 0.1 | 0.104 | 104.0 | 4.38 |
| 1 | 0.991 | 99.1 | 4.47 | |
| 2 | 1.946 | 97.3 | 3.45 | |
| Rain water | 0.1 | 0.0982 | 98.2 | 3.24 |
| 1 | 1.031 | 103.1 | 4.43 | |
| 2 | 2.026 | 101.3 | 4.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Dai, H.; Zeng, Y.; Yang, Y.; Wang, H.; Zhu, X.; Li, L.; Zhou, G.; Chen, R.; Guo, L. A Cross-Linker-Based Poly(Ionic Liquid) for Sensitive Electrochemical Detection of 4-Nonylphenol. Nanomaterials 2019, 9, 513. https://doi.org/10.3390/nano9040513
Hu J, Dai H, Zeng Y, Yang Y, Wang H, Zhu X, Li L, Zhou G, Chen R, Guo L. A Cross-Linker-Based Poly(Ionic Liquid) for Sensitive Electrochemical Detection of 4-Nonylphenol. Nanomaterials. 2019; 9(4):513. https://doi.org/10.3390/nano9040513
Chicago/Turabian StyleHu, Jian, Hao Dai, Yanbo Zeng, Yiwen Yang, Hailong Wang, Xudong Zhu, Lei Li, Guobao Zhou, Ruoyu Chen, and Longhua Guo. 2019. "A Cross-Linker-Based Poly(Ionic Liquid) for Sensitive Electrochemical Detection of 4-Nonylphenol" Nanomaterials 9, no. 4: 513. https://doi.org/10.3390/nano9040513
APA StyleHu, J., Dai, H., Zeng, Y., Yang, Y., Wang, H., Zhu, X., Li, L., Zhou, G., Chen, R., & Guo, L. (2019). A Cross-Linker-Based Poly(Ionic Liquid) for Sensitive Electrochemical Detection of 4-Nonylphenol. Nanomaterials, 9(4), 513. https://doi.org/10.3390/nano9040513