Preparation of High Refractive Index Composite Films Based on Titanium Oxide Nanoparticles Hybridized Hydrophilic Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Composites
2.3. Measurements
3. Results and Discussion
3.1. Characterization of Titanium Oxide Nanoparticles
3.2. Preparation of Hydrophilic Polymer Composite Films Containing TiNP
3.3. Compatibility of TiNPs with the pHEAAm Polymer
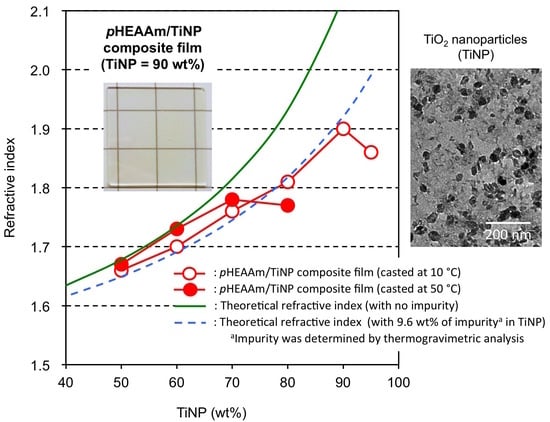
3.4. Refractive Indices of the TiNP/pHEAAm Composite Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lüab, C.; Yang, B. High Refractive Index Organic–inorganic Nanocomposites: Design, Synthesis and Application. J. Mater. Chem. 2009, 19, 2884–2901. [Google Scholar] [CrossRef]
- Hasan, N.; Karkhanis, M.; Ghosh, C.; Khan, F.; Ghosh, T.; Kim, H.; Mastrangelo, C.H. Lightweight Smart Autofocusing Eyeglasses. Proc. SPIE 2018, 1054507. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Montelongo, Y.; Butt, H. Rewritable three-dimensional holographic data storage via optical forces. Appl. Phys. Lett. 2016, 109, 061106. [Google Scholar] [CrossRef]
- Kim, K.C. Effective graded refractive-index anti-reflection coating for high refractive-index polymer ophthalmic lenses. Mater. Lett. 2015, 160, 158–161. [Google Scholar] [CrossRef]
- Li, X.; Yu, X.; Han, Y. Polymer thin films for antireflection coatings. J. Mater. Chem. C 2013, 1, 2266–2285. [Google Scholar] [CrossRef]
- Sanders, D.P. Advances in Patterning Materials for 193 nm Immersion Lithography. Chem. Rev. 2010, 110, 321–360. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, E.K.; Shaver, M.P. Intrinsic high refractive index polymers. Polym. Int. 2014, 64, 6–14. [Google Scholar] [CrossRef]
- Higashihara, T.; Ueda, M. Recent Progress in High Refractive Index Polymers. Macromolecules 2015, 48, 1915–1929. [Google Scholar] [CrossRef]
- Fritsch, J.; Mansfeld, D.; Mehring, M.; Wursche, R.; Grothe, J.; Kaskel, S. Refractive Index Tuning of Highly Transparent Bismuth Containing Polymer Composites. Polymer 2011, 52, 3263–3268. [Google Scholar] [CrossRef]
- Palik, E.D. Handbook of Optical Constants of Solids, 3rd ed.; Academic Press: Orlando, FL, USA, 1985; ISBN 978-0-08-054721-3. [Google Scholar]
- Caseri, W. Nanocomposites of Polymers and Metals or Semiconductors: Historical Background and Optical Properties. Macromol. Rapid Commun. 2000, 21, 705–722. [Google Scholar] [CrossRef]
- Olshavsky, M.A.; Allcock, H.R. Polyphosphazenes with High Refractive Indices: Synthesis, Characterization, and Optical Properties. Macromolecules 1995, 28, 6188–6197. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, C.; Wang, J.X.; Wang, D.; Zeng, X.F.; Chen, J.F. Synthesis of Transparent Aqueous ZrO2 Nanodispersion with a Controllable Crystalline Phase without Modification for a High-Refractive-Index Nanocomposite Film. Langmuir 2018, 34, 6806–6813. [Google Scholar] [CrossRef]
- Tao, P.; Li, Y.; Rungta, A.; Viswanath, A.; Gao, J.; Benicewicz, B.C.; Siegel, R.W.; Schadler, L.S. TiO2 Nanocomposites with High Refractive Index and Transparency. J. Mater. Chem. 2011, 21, 18623–18629. [Google Scholar] [CrossRef]
- Dan, S.; Gu, H.; Tan, J.; Zhang, B.; Zhang, Q. Transparent Epoxy/TiO2 Optical Hybrid Films with Tunable Refractive Index Prepared Via a Simple and Efficient Way. Prog. Org. Coat. 2018, 120, 252–259. [Google Scholar] [CrossRef]
- Imai, Y.; Terahara, A.; Hakuta, Y.; Matsui, K.; Hayashi, H.; Ueno, N. Transparent Poly(bisphenol A Carbonate)-Based Nanocomposites with High Refractive Index Nanoparticles. Eur. Polym. J. 2009, 5, 630–638. [Google Scholar] [CrossRef]
- Nakayama, N.; Hayashi, T. Preparation and Characterization of TiO2 and Polymer Nanocomposite Films with High Refractive Index. J. Appl. Polym. Sci. 2007, 6, 3662–3672. [Google Scholar] [CrossRef]
- Chieh, M.T.; Sheng, H.H.; Chun, C.H.; Yu, C.T.; Hsin, C.T.; Chung, A.W.; Wei, F.S. High Refractive Index Transparent Nanocomposites Prepared by In-situ Polymerization. J. Mater. Chem. C 2014, 2, 2251–2258. [Google Scholar] [CrossRef]
- Cai, B.; Kaino, T.; Sugihara, O. Sulfonyl-Containing Polymer and Its Alumina Nanocomposite with High Abbe Number and High Refractive Index. Opt. Mater. Exp. 2015, 5, 1210–1216. [Google Scholar] [CrossRef]
- Wang, C.; Cui, Q.; Wang, X.; Li, L. Preparation of Hybrid Gold/Polymer Nanocomposites and Their Application in a Controlled Antibacterial Assay. ACS Appl. Mater. Interfaces 2016, 8, 29101–29109. [Google Scholar] [CrossRef]
- Voitylov, A.V.; Veso, O.S.; Petrov, M.P.; Rolich, V.I. Light Refraction in Aqueous Suspensions of Diamond Particles. Colloids Surf. A Physicochem. Eng. Asp. 2017, 538, 417–422. [Google Scholar] [CrossRef]
- Morimune, S.; Kotera, M.; Nishino, T.; Goto, K.; Hata, K. Poly(vinyl alcohol) Nanocomposites with Nanodiamond. Macromolecules 2011, 44, 4415–4421. [Google Scholar] [CrossRef]
- Tristan, S.K.; Ngoc, A.N.; Laura, E.A.; Soha, N.; Edward, A.L.; Sasaan, A.S.; Philip, T.D.; Clay, B.A.; Michael, S.M.; Jim, S.; et al. High Refractive Index Copolymers with Improved Thermomechanical Properties Via the Inverse Vulcanization of Sulfur and 1,3,5-Triisopropenylbenzene. ACS Macro Lett. 2016, 5, 1152–1156. [Google Scholar] [CrossRef]
- Liua, B.T.; Tanga, S.J.; Yub, Y.Y.; Linc, S.H. High-refractive-index Polymer/Inorganic Hybrid Films Containing High TiO2 Contents. Colloids Surf. A Physicochem. Eng. Asp. 2011, 377, 138–143. [Google Scholar] [CrossRef]
- Suac, H.W.; Chen, W.C. High Refractive Index Polyimide–nanocrystalline-titania Hybrid Optical Materials. J. Mater. Chem. 2008, 18, 1139–1145. [Google Scholar] [CrossRef]
- Lu, C.; Cui, Z.; Guan, C.; Guan, J.; Yang, B.; Shen, J. Research on Preparation, Structure and Properties of TiO2/Polythiourethane Hybrid Optical Films with High Refractive Index. Macromol. Mater. Eng. 2003, 288, 717–723. [Google Scholar] [CrossRef]
- Lee, L.H.; Chen, W.C. High-Refractive-Index Thin Films Prepared from Trialkoxysilane-Capped Poly(methyl methacrylate)−Titania Materials. Chem. Mater. 2001, 13, 1137–1142. [Google Scholar] [CrossRef]
- Jintoku, H.; Ihara, H. The Simplest Method for Fabrication of High Refractive Index Polymer–metal Oxide Hybrids Based on a Soap-free Process. Chem. Commun. 2014, 50, 10611–10614. [Google Scholar] [CrossRef]
- Ishii, T.; Hoashi, Y.; Matsumoto, S.; Kuroki, M.; Jintoku, H.; Ogata, T.; Kuwahara, Y.; Takafuji, M.; Nagaoka, S.; Ihara, H. Facile Preparation of Transparent and High Refractive Index Polymer Composites by Polymerization of Monomer–Silicotungstic Acid Mixtures. Chem. Lett. 2017, 46, 489–491. [Google Scholar] [CrossRef]
- Herráez, J.V.; Belda, R. Refractive Indices, Densities and Excess Molar Volumes of Monoalcohols + Water. J. Solut. Chem. 2006, 35, 1315–1328. [Google Scholar] [CrossRef]






| Composition TiNP:pHEAAm | TiNP-1 | TiNP-2 | TiNP-3 | ||||
|---|---|---|---|---|---|---|---|
| 10 °C a | 25 °C a | 50 °C a | 10 °C a | 50 °C a | 10 °C a | 50 °C a | |
| 95:5 | 1.86 | - | - | - | - | - | - |
| 90:10 | 1.90 | 1.79 | - | - | - | - | - |
| 80:20 | 1.81 | 1.78 | 1.77 | - | - | - | - |
| 70:30 | 1.76 | 1.75 | 1.78 | 1.74 | 1.70 | - | - |
| 60:40 | 1.70 | 1.72 | 1.73 | 1.71 | 1.64 | 1.78 | - |
| 50:50 | 1.66 | 1.67 | 1.67 | 1.67 | 1.61 | 1.72 | 1.72 |
| 0:100 | 1.53 | - | - | 1.53 | - | 1.53 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takafuji, M.; Kajiwara, M.; Hano, N.; Kuwahara, Y.; Ihara, H. Preparation of High Refractive Index Composite Films Based on Titanium Oxide Nanoparticles Hybridized Hydrophilic Polymers. Nanomaterials 2019, 9, 514. https://doi.org/10.3390/nano9040514
Takafuji M, Kajiwara M, Hano N, Kuwahara Y, Ihara H. Preparation of High Refractive Index Composite Films Based on Titanium Oxide Nanoparticles Hybridized Hydrophilic Polymers. Nanomaterials. 2019; 9(4):514. https://doi.org/10.3390/nano9040514
Chicago/Turabian StyleTakafuji, Makoto, Maino Kajiwara, Nanami Hano, Yutaka Kuwahara, and Hirotaka Ihara. 2019. "Preparation of High Refractive Index Composite Films Based on Titanium Oxide Nanoparticles Hybridized Hydrophilic Polymers" Nanomaterials 9, no. 4: 514. https://doi.org/10.3390/nano9040514
APA StyleTakafuji, M., Kajiwara, M., Hano, N., Kuwahara, Y., & Ihara, H. (2019). Preparation of High Refractive Index Composite Films Based on Titanium Oxide Nanoparticles Hybridized Hydrophilic Polymers. Nanomaterials, 9(4), 514. https://doi.org/10.3390/nano9040514