Impact of pH on Regulating Ion Encapsulation of Graphene Oxide Nanoscroll for Pressure Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of GO Solution with Various pH
2.3. Preparation of GONS and rGONS
2.4. Encapsulation of Nanoparticles into GONS
2.5. Fabrication of a Device Based on rGONS Mesh and Pressure Sensing Test
2.6. Characterization
3. Results and Discussion
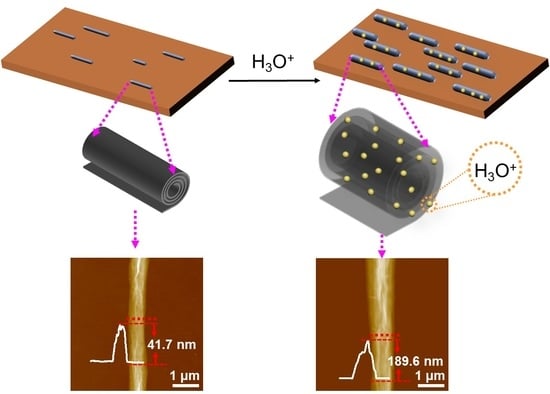
3.1. Effect of pH on Encapsulating H3O+ in GONS
3.2. De-Encapsulation of H3O+ from GONS at High Temperature
3.3. Encapsulation of Other Ions in GONS
3.4. Pressure Sensing Performance of the Device Based on rGONS Mesh
3.5. Discussion of the pH Regulation on the Encapsulation of Ions in GONS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pang, Y.; Tian, H.; Tao, L.; Li, Y.; Wang, X.; Deng, N.; Yang, Y.; Ren, T.L. Flexible, Highly Sensitive, and Wearable Pressure and Strain Sensors with Graphene Porous Network Structure. ACS Appl. Mater. Interfaces 2016, 8, 26458–26462. [Google Scholar] [CrossRef]
- Samad, Y.A.; Li, Y.; Schiffer, A.; Alhassan, S.M.; Liao, K. Graphene Foam Developed with A Novel Two-Step Technique for Low and High Strains and Pressure-Sensing Applications. Small 2015, 11, 2380–2385. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Shu, Y.; Wang, X.F.; Mohammad, M.A.; Bie, Z.; Xie, Q.Y.; Li, C.; Mi, W.T.; Yang, Y.; Ren, T.L. A Graphene-Based Resistive Pressure Sensor with Record-High Sensitivity in A Wide Pressure Range. Sci. Rep. 2015, 5, 8603. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Chen, S.; Wang, L.; Jiang, K.; Shen, G. An Ultra-Sensitive and Rapid Response Speed Graphene Pressure Sensors for Electronic Skin and Health Monitoring. Nano Energy 2016, 23, 7–14. [Google Scholar] [CrossRef]
- Qi, K.; He, J.; Wang, H.; Zhou, Y.; You, X.; Nan, N.; Shao, W.; Wang, L.; Ding, B.; Cui, S. A Highly Stretchable Nanofiber-Based Electronic Skin with Pressure-, Strain-, and Flexion-Sensitive Properties for Health and Motion Monitoring. ACS Appl. Mater. Interfaces 2017, 9, 42951–42960. [Google Scholar] [CrossRef]
- Peng, X.; Wu, K.; Hu, Y.; Zhuo, H.; Chen, Z.; Jing, S.; Liu, Q.; Liu, C.; Zhong, L. A Mechanically Strong and Sensitive CNT/rGO–CNF Carbon Aerogel for Piezoresistive Sensors. J. Mater. Chem. A 2018, 6, 23550–23559. [Google Scholar] [CrossRef]
- Savoskin, M.V.; Mochalin, V.N.; Yaroshenko, A.P.; Lazareva, N.I.; Konstantinova, T.E.; Barsukov, I.V.; Prokofiev, I.G. Carbon Nanoscrolls Produced from Acceptor-Type Graphite Intercalation Compounds. Carbon 2007, 45, 2797–2800. [Google Scholar] [CrossRef]
- Viculis, L.M.; Mack, J.J.; Kaner, R.B. A Chemical Route to Carbon Nanoscrolls. Science 2003, 299, 1361. [Google Scholar] [CrossRef] [PubMed]
- Mpourmpakis, G.; Tylianakis, E.; Froudakis, G.E. Carbon Nanoscrolls: A Promising Material for Hydrogen Storage. Nano Lett. 2007, 7, 1893–1897. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Ju, L.; Feng, X.; Sun, Y.; Zhou, R.; Liu, K.; Fan, S.; Li, Q.; Jiang, K. Controlled Fabrication of High-Quality Carbon Nanoscrolls from Monolayer Graphene. Nano Lett. 2009, 9, 2565–2570. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, X.; Xu, H.; Zou, Y.; Gu, R.; Xu, M.; Jen, A.K.Y.; Chen, H. Highly-Efficient Fabrication of Nanoscrolls from Functionalized Graphene Oxide by Langmuir–Blodgett Method. Carbon 2010, 48, 4475–4482. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Min, D.-H. Preparation of Scrolled Graphene Oxides with Multi-Walled Carbon Nanotube Templates. Carbon 2010, 48, 4283–4288. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Zhang, H.; Ran, F.; Yang, P.; Li, H. Graphene Oxide Scroll Meshes Encapsulated Ag Nanoparticles for Humidity Sensing. RSC Adv. 2017, 7, 40119–40123. [Google Scholar] [CrossRef]
- Wang, L.; Yang, P.; Liu, Y.; Fang, X.; Shi, X.; Wu, S.; Huang, L.; Li, H.; Huang, X.; Huang, W. Scrolling up Graphene Oxide Nanosheets Assisted by sSlf-Assembled Monolayers of Alkanethiols. Nanoscale 2017, 9, 9997–10001. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, J.; Chen, T.; Tan, K.S.; Jia, X.; Luo, Z.; Cong, C.; Yang, H.; Li, C.M.; Yu, T. Fabrication of Co3O4-Reduced Graphene Oxide Scrolls for High-Performance Supercapacitor Electrodes. Phys. Chem. Chem. Phys. 2011, 13, 14462–14465. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Kuang, Y.; Liu, G.; Liu, R.; Huang, Z.; Fu, C.; Zhou, H. Supercapacitors Based on High-Quality Graphene Scrolls. Nanoscale 2012, 4, 3997–4001. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Qi, X.; He, Q.; Liusman, C.; Lu, G.; Zhou, X.; Zhang, H. Graphene Oxide Scrolls on Hydrophobic Substrates Fabricated by Molecular Combing and Their Application in Gas Sensing. Small 2013, 9, 382–386. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, B.; Chen, J.; Gao, C. Highly Efficient Synthesis of Neat Graphene Nanoscrolls from Graphene Oxide by Well-Controlled Lyophilization. Chem. Mater. 2014, 26, 6811–6818. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, B.; Zheng, Z.; Yang, J.; Yang, Z.; Zhang, P.; Ren, W.; Yan, X. Facile Preparation of One-Dimensional Wrapping Structure: Graphene Nanoscroll-Wrapped of Fe3O4 Nanoparticles and Its Application for Lithium-Ion Battery. ACS Appl. Mater. Interfaces 2014, 6, 9890–9896. [Google Scholar] [CrossRef] [PubMed]
- Rani, J.R.; Oh, S.-I.; Woo, J.M.; Tarwal, N.L.; Kim, H.-W.; Mun, B.S.; Lee, S.; Kim, K.-J.; Jang, J.-H. Graphene Oxide–Phosphor Hybrid Nanoscrolls with High Luminescent Quantum Yield: Synthesis, Structural, and X-ray Absorption Studies. ACS Appl. Mater. Interfaces 2015, 7, 5693–5700. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Gao, X.; Wang, B.; Liu, H.; Wu, H.; Liu, H.; Dou, S. Nitrogen-Doped Graphene Ribbon Assembled Core-Sheath MnO@Graphene Scrolls as Hierarchically Ordered 3D Porous Electrodes for Fast and Durable Lithium Storage. Adv. Funct. Mater. 2016, 26, 7754–7765. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.; Xu, Z.; Gao, C. Mass Production of Graphene Nanoscrolls and tTeir Application in High Rate Performance Supercapacitors. Nanoscale 2016, 8, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, H.; Qi, X.; He, Q.; Xu, B.; Zhang, H. Graphene Oxide Architectures Prepared by Molecular Combing on Hydrophilic-hydrophobic Micropatterns. Small 2014, 10, 2239–2244. [Google Scholar] [CrossRef]
- Fan, T.; Zeng, W.; Niu, Q.; Tong, S.; Cai, K.; Liu, Y.; Huang, W.; Min, Y.; Epstein, A.J. Fabrication of High-Quality Graphene Oxide Nanoscrolls and Application in Supercapacitor. Nanoscale Res. Lett. 2015, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Meng, L.; Zhang, W.; Liu, W.; Zhang, L.; Zhang, Y. Facile Preparation of Nitrogen-Doped Graphene Scrolls Via Acoustic Cavitation as Electrocatalyst for Glucose Biosensing. J. Solid State Electrochem. 2015, 20, 439–447. [Google Scholar] [CrossRef]
- Wu, J.; Yang, J.; Huang, Y.; Li, H.; Fan, Z.; Liu, J.; Cao, X.; Huang, X.; Huang, W.; Zhang, H. Graphene Oxide Scroll Meshes Prepared by Molecular Combing for Transparent and Flexible Electrodes. Adv. Mater. Technol. 2017, 2, 1600231. [Google Scholar] [CrossRef]
- Yan, M.; Wang, F.; Han, C.; Ma, X.; Xu, X.; An, Q.; Xu, L.; Niu, C.; Zhao, Y.; Tian, X.; et al. Nanowire Templated Semihollow Bicontinuous Graphene Scrolls: Designed Construction, Mechanism, and Enhanced Energy Storage Performance. J. Am. Chem. Soc. 2013, 135, 18176–18182. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Li, T.; Zhong, Q.; Li, H.; Huang, J. Graphene Nanoscrolls Encapsulated TiO2 (B) Nanowires for Lithium Storage. J. Power Sources 2014, 268, 372–378. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Chen, J. Ultrasonic Cavitation Assisted Hydrogen Implosion Synthesis of Pt Nanoparticles/Nitrogen-Doped Graphene Nanohybrid Scrolls and Their Electrocatalytic Oxidation of Methanol. Ionics 2014, 21, 1287–1294. [Google Scholar] [CrossRef]
- Meng, L.; Xia, Y.; Liu, W.; Zhang, L.; Zou, P.; Zhang, Y. Hydrogen Microexplosion Synthesis of Platinum Nanoparticles/Nitrogen Doped Graphene Nanoscrolls as New Amperometric Glucose Biosensor. Electrochim. Acta 2015, 152, 330–337. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Wang, H.; Tang, H.; Xu, L.; Li, H.; Zhang, L. Preparation of Nanoscrolls by Rolling up Graphene Oxide-Polydopamine-Au Sheets using Lyophilization Method. Chem. Asian J. 2016, 11, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Lee, J.; Kim, J.M.; Seong, C.-Y.; Seong, K.-d.; Piao, Y. Well-Dispersed Sulfur Wrapped in Reduced Graphene Oxide Nanoscroll as Cathode Material for Lithium–Sulfur Battery. J. Electroanal. Chem. 2016, 780, 19–25. [Google Scholar] [CrossRef]
- Zheng, B.-N.; Gao, C. Preparation of Graphene Nanoscroll/Polyaniline Composites and Their Use in High Performance Supercapacitors. New Carbon Mater. 2016, 31, 315–320. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of Graphene Oxide Via L-ascorbic Acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Merino, M.J.; Guardia, L.; Paredes, J.I.; Villar-Rodil, S.; Solís-Fernández, P.; Martínez-Alonso, A.; Tascón, J.M.D. Vitamin C Is an Ideal Substitute for Hydrazine in the Reduction of Graphene Oxide Suspensions. J. Phys. Chem. C 2010, 114, 6426–6432. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Huang, X.; Yin, Z.; Liu, J.; Zhang, H. A Universal, Rapid Method for Clean Transfer of Nanostructures onto Various Substrates. ACS Nano 2014, 8, 6563–6570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, F.; Cote, L.J.; Huang, J. Graphene Oxide: Furface Activity and Two-Dimensional Assembly. Adv. Mater. 2010, 22, 1954–1958. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of Graphite Oxide Revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Wang, L.; Pei, C.; Wei, C.; You, H.; Zhang, J.; Li, H. Impact of pH on Regulating Ion Encapsulation of Graphene Oxide Nanoscroll for Pressure Sensing. Nanomaterials 2019, 9, 548. https://doi.org/10.3390/nano9040548
Zhao W, Wang L, Pei C, Wei C, You H, Zhang J, Li H. Impact of pH on Regulating Ion Encapsulation of Graphene Oxide Nanoscroll for Pressure Sensing. Nanomaterials. 2019; 9(4):548. https://doi.org/10.3390/nano9040548
Chicago/Turabian StyleZhao, Weihao, Lin Wang, Chengjie Pei, Cong Wei, Hui You, Jindong Zhang, and Hai Li. 2019. "Impact of pH on Regulating Ion Encapsulation of Graphene Oxide Nanoscroll for Pressure Sensing" Nanomaterials 9, no. 4: 548. https://doi.org/10.3390/nano9040548
APA StyleZhao, W., Wang, L., Pei, C., Wei, C., You, H., Zhang, J., & Li, H. (2019). Impact of pH on Regulating Ion Encapsulation of Graphene Oxide Nanoscroll for Pressure Sensing. Nanomaterials, 9(4), 548. https://doi.org/10.3390/nano9040548