Microscopic Views of Atomic and Molecular Oxygen Bonding with epi Ge(001)-2 × 1 Studied by High-Resolution Synchrotron Radiation Photoemission
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparations
2.2. Data Analysis
3. Results and Discussion
3.1. Clean epi Ge(001)-2 × 1 Surface
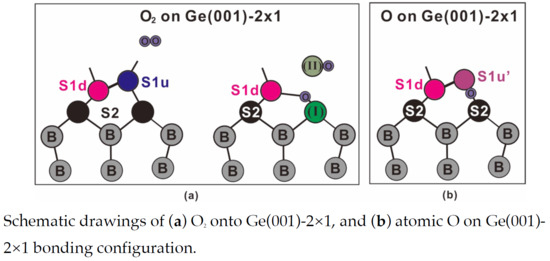
3.2. Adsorption of Molecular Oxygen on epi Ge(001)-2 × 1 Surface
3.3. Adsorption of Atomic Oxygen on epi Ge(001)-2 × 1 Surface
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chu, L.K.; Chu, R.L.; Lin, T.D.; Lee, W.C.; Lin, C.A.; Huang, M.L.; Lee, Y.J.; Kwo, J.; Hong, M. Effective passivation and high-performance metal-oxide-semiconductor devices using ultra-high-vacuum deposited high-ĸ dielectrics on Ge without interfacial layers. Solid-State Electron. 2010, 54, 965. [Google Scholar] [CrossRef]
- Chu, R.L.; Liu, Y.C.; Lee, W.C.; Lin, T.D.; Huang, M.L.; Pi, T.W.; Kwo, J.; Hong, M. Greatly improved interfacial passivation of in-situ high ĸ dielectric deposition on freshly grown molecule beam epitaxy Ge epitaxial layer on Ge(100). Appl. Phys. Lett. 2014, 104, 202102. [Google Scholar]
- Dimoulas, A.; Tsoutsou, D.; Galata, S.; Panayiotatos, Y.; Mavrou, G.; Golias, E. Ge surfaces and its passivation by rare earth lanthanum germanate dielectric. ECS Trans. 2010, 33, 433–446. [Google Scholar]
- Hu, S.; McDaniel, M.D.; Posadas, A.; Hu, C.; Wu, H.; Yu, E.T.; Smith, D.J.; Demkov, A.A.; Ekerdt, J.G. Monolithic integration of perovskites on Ge(001) by atomic layer deposition: A case study with SrHfxTi1−xO3. MRS Commun. 2016, 6, 125–132. [Google Scholar] [CrossRef]
- Lu, C.; Lee, C.H.; Zhang, W.; Nishimura, T.; Nagashio, K.; Toriumi, A. Structural and thermodynamic consideration of metal oxide doped GeO2 for gate stack formation on germanium. J. Appl. Phys. 2014, 116, 174103. [Google Scholar] [CrossRef]
- Pourtois, G.; Houssa, M.; Delabie, A.; Conard, T.; Caymax, M.; Meuris, M.; Heyns, M.M. Ge 3d core-level shifts at (100)Ge/Ge(Hf)O2 interfaces: A first-principles investigation. Appl. Phys. Lett. 2008, 92, 032105. [Google Scholar] [CrossRef]
- Sun, J.; Lu, J. Interface engineering and gate dielectric engineering for high performance Ge MOSFETs. Adv. Cond. Matter Phys. 2015, 2015, 639218. [Google Scholar] [CrossRef]
- Takagi, S.; Kim, S.H.; Yokoyama, M.; Nishi, K.; Zhang, R.; Takenaka, M. Material challenges and opportunities in Ge/III-V channel MOSFETs. ECS Trans. 2014, 64, 99–110. [Google Scholar] [CrossRef]
- Hong, M.; Kwo, J.; Lin, T.D.; Huang, M.L. InGaAs metal oxide semiconductor devices with Ga2O3(Gd2O3) high-ĸ dielectrics for science and technology beyond Si CMOS. MRS Bull. 2009, 34, 514–521. [Google Scholar] [CrossRef]
- Lin, T.D.; Chang, Y.H.; Lin, C.A.; Huang, M.L.; Lee, W.C.; Kwo, J.; Hong, M. Realization of high-quality HfO2 on In0.53Ga0.47As by in-situ atomic-layerdeposition. Appl. Phys. Lett. 2012, 100, 172110. [Google Scholar]
- Hong, M.; Wan, H.W.; Lin, K.Y.; Chang, Y.C.; Chen, M.H.; Lin, Y.H.; Lin, T.D.; Pi, T.W.; Kwo, J. Perfecting the Al2O3/In0.53Ga0.47As interfacial electronic structure in pushing metal-oxide-semiconductor field-effect-transistor device limits using in-situ atomic-layer-deposition. Appl. Phys. Lett. 2017, 111, 123502. [Google Scholar] [CrossRef]
- Lin, T.D.; Chiu, H.C.; Chang, P.; Tung, L.T.; Chen, C.P.; Hong, M.; Kwo, J.; Tsai, W.; Wang, Y.C. High-performance self-aligned inversion-channel In0.53Ga0.47As metal-oxide-semiconductor field-effect-transistor with Al2O3/Ga2O3(Gd2O3) as gate dielectrics. Appl. Phys. Lett. 2008, 93, 033516. [Google Scholar] [CrossRef]
- Ren, F.; Kuo, J.M.; Hong, M.; Hobson, W.S.; Lothian, J.R.; Lin, J.; Tsai, H.S.; Mannaerts, J.P.; Kwo, J.; Chu, S.N.G.; et al. Ga2O3(Gd2O3)/InGaAs enhancement-mode n-channel MOSFETs. IEEE Electron Device Lett. 1998, 19, 309–311. [Google Scholar] [CrossRef]
- Sun, S.; Sun, Y.; Liu, Z.; Lee, D.-I.; Peterson, S.; Pianetta, P. Surface termination and roughness of Ge(100) cleaned by HF and HCl solutions. Appl. Phys. Lett. 2006, 88, 021903. [Google Scholar] [CrossRef]
- Kim, H.; McIntyre, P.C.; Chui, C.O.; Saraswat, K.C.; Cho, M.-H. Interfacial characteristics of HfO2 grown on nitrided Ge(100) substrates by atomic-layer deposition. Appl. Phys. Lett. 2004, 85, 2902. [Google Scholar] [CrossRef]
- Lee, W.C.; Chin, B.H.; Chu, L.K.; Lin, T.D.; Lee, Y.J.; Tung, L.T.; Lee, C.H.; Hong, M.; Kwo, J. Molecular beam epitaxy-grown Al2O3/HfO2 high-ĸ dielectrics for germanium. J. Cryst. Growth 2009, 311, 2187–2190. [Google Scholar] [CrossRef]
- Chu, L.K.; Lin, T.D.; Huang, M.L.; Chu, R.L.; Chang, C.C.; Kwo, J.; Hong, M. Ga2O3(Gd2O3) on Ge without interfacial layers: Energy-band parameters and metal oxide semiconductor devices. Appl. Phys. Lett. 2009, 94, 202108. [Google Scholar] [CrossRef]
- Delabie, A.; Bellenger, F.; Houssa, M.; Conard, T.; Elshocht, S.V.; Caymax, M.; Heyns, M.; Meuris, M. Effective electrical passivation of Ge(100) for high-ĸ gate dielectric layers using germanium oxide. Appl. Phys. Lett. 2007, 91, 082904. [Google Scholar] [CrossRef]
- Xie, Q.; Deng, S.; Schaekers, M.; Lin, D.; Caymax, M.; Delabie, A.; Jiang, Y.; Qu, X.; Dedutytsche, D.; Detavernier, C. High-performance Ge MOS capacitors by O2 plasma passivation and O2 ambient annealing. IEEE Electron Device Lett. 2011, 32, 1656. [Google Scholar] [CrossRef]
- Lee, C.H.; Nishimura, T.; Nagashio, K.; Kita, K.; Toriumi, A. High-electron-mobility Ge/GeO2 n-MOSFETs with two-step oxidation. IEEE Trans. Electron Devices 2011, 58, 1295. [Google Scholar]
- Pi, T.W.; Cheng, C.P.; Wertheim, G.K. The reaction of Si(001)-2 × 1 with magnasium. J. Appl. Phys. 2011, 109, 043701. [Google Scholar]
- Pi, T.W.; Hong, I.H.; Cheng, C.P.; Wertheim, G.K. Surface photoemission from Si(100) and inelastic electron mean-free-path in silicon. J. Electron Spectrosc. Relat. Phenom. 2000, 107, 163–176. [Google Scholar] [CrossRef]
- Pi, T.W.; Wen, J.F.; Ouyang, C.P.; Wu, R.T. Surface core-level shifts of Ge(100)-2 × 1. Phys. Rev. B 2001, 63, 153310. [Google Scholar] [CrossRef]
- Pi, T.W.; Wen, J.F.; Ouyang, C.P.; Wu, R.T.; Wertheim, G.K. Oxidation of Si(001)-2 × 1. Surf. Sci. 2001, 478, L333–L338. [Google Scholar] [CrossRef]
- Wada, A.; Zhang, R.; Takagi, S.; Samukawa, S. Formation of thin germanium dioxide film with a high-quality interface using a direct neutral beam oxidation process. Jpn. J. App. Phys. 2012, 51, 125603. [Google Scholar] [CrossRef]
- Sahari, S.K.; Murakami, H.; Fujioka, T.; Bando, T.; Ohta, A.; Makihara, K.; Higashi, S.; Miyazaki, S. Native oxidation growth on Ge(111) and (100) surfaces. Jpn. J. App. Phys. 2011, 50, 04DA12. [Google Scholar] [CrossRef]
- Prabhakaran, K.; Maeda, F.; Watanabe, Y.; Ogino, T. Distinctly different thermal decomposition pathways of ultrathin oxide layer on Ge and Si surfaces. Appl. Phys. Lett. 2000, 76, 2244. [Google Scholar] [CrossRef]
- Milojevic, M.; Contreras-Guerrero, R.; Lopez-Lopez, M.; Kim, J.; Wallace, R.M. Characterization of the “clean-up” of the oxidized Ge(100) surface by atomic layer deposition. Appl. Phys. Lett. 2009, 95, 212902. [Google Scholar] [CrossRef]
- Molle, A.; Spiga, S.; Fanciulli, M. Stability and interface quality of GeO2 films grown on Ge by atomic oxygen assisted deposition. J. Chem. Phys. 2008, 129, 011104. [Google Scholar] [CrossRef]
- Molle, A.; Bhuiyan, M.N.K.; Tallarida, G.; Fanciulli, M. In situ chemical and structural Investigations of the oxidation of Ge(001) substrates by atomic oxygen. Appl. Phys. Lett. 2006, 89, 083504. [Google Scholar] [CrossRef]
- Yoshigoe, A.; Teraoka, Y.; Okada, R.; Yamada, Y.; Sasaki, M. In situ synchrotron radiation photoelectron spectroscopy study of the oxidation of the Ge(001)-2 × 1 surface by supersonic molecular oxygen beams. J. Chem. Phys. 2014, 141, 174708. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, K.S.; Morikawa, Y. Structure and binding energies of unsaturated hydrocarbons on Si(001) and Ge(001). J. Chem. Phys. 2006, 124, 024716. [Google Scholar] [CrossRef]
- Fan, X.L.; Lau, W.M.; Liu, Z.F. Nondissociative Adsorption of O2 on Ge(100). J. Phys. Chem. C 2009, 113, 8786–8793. [Google Scholar] [CrossRef]
- Fleischmann, C.; Schouteden, K.; Merckling, C.; Sioncke, S.; Meuris, M.; Haesendonck, C.V.; Temst, K.; Vantomme, A. Adsorption of O2 on Ge(100): Atomic geometry and site-specific electronic structure. J. Phys. Chem. C 2012, 116, 9925–9929. [Google Scholar] [CrossRef]
- Pi, T.W.; Lin, Y.H.; Fanchiang, Y.T.; Chiang, T.H.; Wei, C.H.; Lin, Y.C.; Wertheim, G.K.; Kwo, J.; Hong, M. In-situ atomic layer deposition of tri-methylaluminum and water on prestine single-crystal (In)GaAs surfaces: Electronic and electric structures. Nanotechnology 2015, 26, 164001. [Google Scholar] [CrossRef]
- Fukuda, T.; Ogino, T. Initial oxygen reaction on Ge(100) 2 × 1 surfaces. Phys. Rev. B 1997, 56, 13190–13193. [Google Scholar] [CrossRef]
- Shah, G.A.; Radny, M.W.; Smith, P.V. Initial stages of oxygen chemisorption on the Ge(001) surface. J. Phys. Chem. C 2014, 118, 15795–15803. [Google Scholar] [CrossRef]
- Soon, J.M.; Lim, C.W.; Loh, K.P.; Ma, N.L.; Wu, P. Initial-stage oxidation mechanism of Ge(100) 2 × 1 dimers. Phys. Rev. B 2005, 72, 115343. [Google Scholar] [CrossRef]
- Franco, J.; Kaczer, B.; Roussel, P.J.; Mitard, J.; Sioncke, S.; Witters, L.; Mertens, H.; Grasser, T.; Groeseneken, G. Understanding the suppresed charge trapping in relaxed- and strained-Ge/SiO2/HfO2 pMOSFETs and implications for the screening of alternative high-mobility substrate/dielectric CMOS gate stacks. In Proceedings of the 2013 IEEE International Electronic Devices Meeting, Washington, DC, USA, 9–11 December 2013; pp. 358–362. [Google Scholar]
- Wan, H.W.; Hong, Y.J.; Cheng, Y.T.; Kwo, J.; Hong, M. BTI Characterization of MBE Si-capped Ge gate stack and defect reduction via forming gas annealing. In Proceedings of the 2019 IEEE International Reliability Symposium, Monterey, CA, USA, 31 March–4 April 2019. [Google Scholar]
- Lee, K.Y.; Lee, W.C.; Lee, Y.J.; Huang, M.L.; Chang, C.H.; Wu, T.B.; Hong, M.; Kwo, J. Molecular beam epitaxy grown template for subsequent atomic layer deposition of high κ dielectrics. Appl. Phys. Lett. 2006, 89, 222906. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Lin, Y.H.; Chen, W.S.; Lin, K.Y.; Wan, H.W.; Cheng, C.P.; Cheng, H.H.; Kwo, J.; Hong, M.; Pi, T.W. Surface electronic structure of epi Germanium (001)-2 × 1. Appl. Phys. Express 2017, 10, 075701. [Google Scholar] [CrossRef]
- Cao, R.; Yang, X.; Terry, J.; Pianetta, P. Core-level shifts of the Ge(100)-(2 × 1) surface and their origins. Phys. Rev. B 1992, 45, 13749–13752. [Google Scholar] [CrossRef]
- Eriksson, P.E.J.; Uhrberg, R.I.G. Surface core-level shifts on clean Si(001) and Ge(001) studied with photoelectron spectroscopy and density functional theory calculations. Phys. Rev. B 2010, 81, 125443. [Google Scholar] [CrossRef]
- Goldoni, A.; Modesti, S.; Dhanak, V.R.; Sancrotti, M.; Santoni, A. Evidence for three surface components in the 3d core-level photoemission spectra of Ge(100)-(2 × 1) surface. Phys. Rev. B 1996, 54, 11340–11345. [Google Scholar] [CrossRef]
- Landemark, E.; Karlsson, C.J.; Johansson, L.S.O.; Uhrberg, R.I.G. Electronic structure of clean and hydrogen-chemisorbed Ge(001) surfaces studied by photoelectron spectroscopy. Phys. Rev. B 1994, 49, 16523–16533. [Google Scholar] [CrossRef]
- Le Lay, G.; Kanski, J.; Nilsson, P.O.; Karlsson, U.O.; Hricovini, K. Surface core-level shifts on Ge(100): c(4 × 2) to 2 × 1 and 1 × 1 phase transitions. Phys. Rev. B 1992, 45, 6692–6699. [Google Scholar] [CrossRef]
- Margaritondo, G.; Rowe, J.E.; Christman, S.B. Photoionization cross section of d-core levels in solids: A synchrotron radiation study of the spin-orbit branching ratio. Phys. Rev. B 1979, 19, 2850–2855. [Google Scholar] [CrossRef]
- Aarts, J.; Hoeven, A.-J.; Larsen, P.K. Core-level study of the phase transition on the Ge(111)-c(2 × 8) surface. Phys. Rev. B 1988, 38, 3925–3930. [Google Scholar] [CrossRef]
- Pehlke, E.; Scheffler, M. Evidence for site-sensitive screening of core holes at the Si and Ge (001) surface. Phys. Rev. Lett. 1993, 71, 2338–2341. [Google Scholar] [CrossRef] [PubMed]
- Binder, J.F.; Broqvist, P.; Komsa, H.; Pasquarello, A. Germanium core-level shifts at Ge/GeO2 interfaces through hybrid functionals. Phys. Rev. B 2012, 85, 245305. [Google Scholar] [CrossRef]
- Schmeisser, D.; Schnell, R.D.; Bogen, A.; Himpsel, F.J.; Rieger, D.; Landgren, G.; Morar, J.F. Surface oxidation states of germanium. Surf. Sci. 1986, 172, 455–465. [Google Scholar] [CrossRef]
- Hansen, D.A.; Hudson, J.B. Oxygen scattering and initial chemisorption probability on Ge(100). Surf. Sci. 1991, 254, 222–234. [Google Scholar] [CrossRef]
- Cheng, C.P.; Chen, W.S.; Lin, K.Y.; Wei, G.J.; Cheng, Y.T.; Lin, Y.H.; Wan, H.W.; Pi, T.W.; Tung, R.T.; Kwo, J.; et al. Atomic nature of the Schottky barrier height formation of the Ag/GaAs(001)-2 × 4 interface: An in-situ synchrotron radiation photoemission study. Appl. Surf. Sci. 2017, 393, 294–298. [Google Scholar] [CrossRef]
- Kronik, L.; Shapira, Y. Surface photovoltage phenomena: Theory, experiment, and applications. Surf. Sci. Rep. 1999, 37, 1–206. [Google Scholar] [CrossRef]
- Radny, M.W.; Shah, G.A.; Schofield, S.R.; Smith, P.V.; Curson, N.J. Valence surface electronic states on Ge(001). Phys. Rev. Lett. 2008, 100, 246807. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.-T.; Wan, H.-W.; Cheng, C.-P.; Kwo, J.; Hong, M.; Pi, T.-W. Microscopic Views of Atomic and Molecular Oxygen Bonding with epi Ge(001)-2 × 1 Studied by High-Resolution Synchrotron Radiation Photoemission. Nanomaterials 2019, 9, 554. https://doi.org/10.3390/nano9040554
Cheng Y-T, Wan H-W, Cheng C-P, Kwo J, Hong M, Pi T-W. Microscopic Views of Atomic and Molecular Oxygen Bonding with epi Ge(001)-2 × 1 Studied by High-Resolution Synchrotron Radiation Photoemission. Nanomaterials. 2019; 9(4):554. https://doi.org/10.3390/nano9040554
Chicago/Turabian StyleCheng, Yi-Ting, Hsien-Wen Wan, Chiu-Ping Cheng, Jueinai Kwo, Minghwei Hong, and Tun-Wen Pi. 2019. "Microscopic Views of Atomic and Molecular Oxygen Bonding with epi Ge(001)-2 × 1 Studied by High-Resolution Synchrotron Radiation Photoemission" Nanomaterials 9, no. 4: 554. https://doi.org/10.3390/nano9040554
APA StyleCheng, Y. -T., Wan, H. -W., Cheng, C. -P., Kwo, J., Hong, M., & Pi, T. -W. (2019). Microscopic Views of Atomic and Molecular Oxygen Bonding with epi Ge(001)-2 × 1 Studied by High-Resolution Synchrotron Radiation Photoemission. Nanomaterials, 9(4), 554. https://doi.org/10.3390/nano9040554