Low-Temperature Vapor-Phase Synthesis of Single-Crystalline Gold Nanostructures: Toward Exceptional Electrocatalytic Activity for Methanol Oxidation Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Au NPs on an FTO Substrate
2.2. Synthesis of Au Nanoplates on an FTO Substrate
2.3. Cyclic Voltammetry (CV) Measurements
2.4. Instrumentation
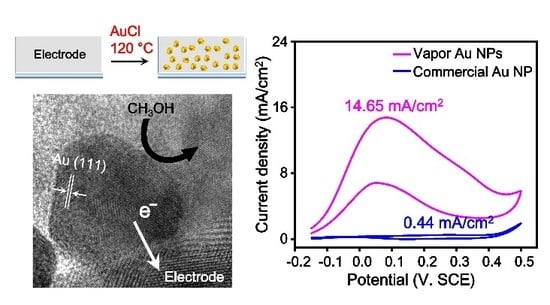
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef] [PubMed]
- Cantale, V.; Simeone, F.C.; Gambari, R.; Rampi, M.A. Gold nano-islands on FTO as plasmonic nanostructures for biosensors. Sens. Actuator B 2011, 152, 206–213. [Google Scholar]
- Diaz-Morales, O.; Calle-Vallejo, F.; de Munck, C.; Koper, M.T. Electrochemical water splitting by gold: Evidence for an oxide decomposition mechanism. Chem. Sci. 2013, 4, 2334–2343. [Google Scholar] [CrossRef]
- Al-Harbi, E.; Aziz, M.; Oyama, M.; El-Naggar, A.; AlZayed, N.; Wojciechowski, A.; Kityk, I. Gold nanoparticles deposited on fluorine-doped tin oxide substrates as materials for laser operated optoelectronic devices. J. Mater. Sci. Mater. Electron. 2013, 24, 2422–2425. [Google Scholar] [CrossRef]
- Talley, C.E.; Jackson, J.B.; Oubre, C.; Grady, N.K.; Hollars, C.W.; Lane, S.M.; Huser, T.R.; Nordlander, P.; Halas, N.J. Surface-enhanced Raman scattering from individual Au nanoparticles and nanoparticle dimer substrates. Nano Lett. 2005, 5, 1569–1574. [Google Scholar] [CrossRef]
- Rodriguez, P.; Kwon, Y.; Koper, M.T. The promoting effect of adsorbed carbon monoxide on the oxidation of alcohols on a gold catalyst. Nat. Chem. 2012, 4, 177. [Google Scholar] [CrossRef]
- Luo, J.; Lou, Y.; Maye, M.M.; Zhong, C.-J.; Hepel, M. An EQCN assessment of electrocatalytic oxidation of methanol at nanostructured Au–Pt alloy nanoparticles. Electrochem. Commun. 2001, 3, 172–176. [Google Scholar] [CrossRef]
- Hernández, J.; Solla-Gullón, J.; Herrero, E.; Aldaz, A.; Feliu, J.M. Methanol oxidation on gold nanoparticles in alkaline media: Unusual electrocatalytic activity. Electrochim. Acta 2006, 52, 1662–1669. [Google Scholar] [CrossRef]
- Borkowska, Z.; Tymosiak-Zielinska, A.; Nowakowski, R. High catalytic activity of chemically activated gold electrodes towards electro-oxidation of methanol. Electrochim. Acta 2004, 49, 2613–2621. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, P.; Ma, H.; Ding, Y. Nanostructured porous gold for methanol electro-oxidation. J. Phys. Chem. C 2007, 111, 10382–10388. [Google Scholar] [CrossRef]
- Yu, C.; Jia, F.; Ai, Z.; Zhang, L. Direct oxidation of methanol on self-supported nanoporous gold film electrodes with high catalytic activity and stability. Chem. Mater. 2007, 19, 6065–6067. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, S.; Lin, Y.; Liu, G. Electrocatalytic performance of gold nanoparticles supported on activated carbon for methanol oxidation in alkaline solution. J. Phys. Chem. C 2011, 115, 6986–6993. [Google Scholar] [CrossRef]
- Jena, B.K.; Raj, C.R. Synthesis of flower-like gold nanoparticles and their electrocatalytic activity towards the oxidation of methanol and the reduction of oxygen. Langmuir 2007, 23, 4064–4070. [Google Scholar] [CrossRef] [PubMed]
- Negishi, Y.; Nakazaki, T.; Malola, S.; Takano, S.; Niihori, Y.; Kurashige, W.; Yamazoe, S.; Tsukuda, T.; Häkkinen, H. A critical size for emergence of nonbulk electronic and geometric structures in dodecanethiolate-protected Au clusters. J. Am. Chem. Soc. 2015, 137, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Cheng, W. Nanoparticle-modified electrode with size-and shape-dependent electrocatalytic activities. Langmuir 2013, 29, 3125–3132. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gong, J.; Xiong, Y.; Yang, J.; Gao, Y.; Liu, Y.; Lu, X.; Tang, Z. Shape-dependent electrocatalytic activity of monodispersed gold nanocrystals toward glucose oxidation. Chem. Commun. 2011, 47, 6894–6896. [Google Scholar] [CrossRef]
- Zhu, M.; Lei, B.; Ren, F.; Chen, P.; Shen, Y.; Guan, B.; Du, Y.; Li, T.; Liu, M. Branched Au nanostructures enriched with a uniform facet: Facile synthesis and catalytic performances. Sci. Rep. 2014, 4, 5259. [Google Scholar] [CrossRef]
- Park, J.Y.; Renzas, J.; Hsu, B.B.; Somorjai, G.A. Interfacial and chemical properties of Pt/TiO2, Pd/TiO2, and Pt/GaN catalytic nanodiodes influencing hot electron flow. J. Phys. Chem. C 2007, 111, 15331–15336. [Google Scholar] [CrossRef]
- Park, J.Y.; Renzas, J.; Contreras, A.; Somorjai, G.A. The genesis and importance of oxide–metal interface controlled heterogeneous catalysis; the catalytic nanodiode. Top. Catal. 2007, 46, 217–222. [Google Scholar] [CrossRef]
- Donoeva, B.G.; Ovoshchnikov, D.S.; Golovko, V.B. Establishing a Au nanoparticle size effect in the oxidation of cyclohexene using gradually changing Au catalysts. ACS Catal. 2013, 3, 2986–2991. [Google Scholar] [CrossRef]
- Elliott, E.W., III; Glover, R.D.; Hutchison, J.E. Removal of thiol ligands from surface-confined nanoparticles without particle growth or desorption. ACS Nano 2015, 9, 3050–3059. [Google Scholar] [CrossRef] [PubMed]
- Muglali, M.I.; Bashir, A.; Rohwerder, M. A study on oxygen reduction inhibition at pyridine-terminated self assembled monolayer modified Au(111) electrodes. Phys. Status Solidi (A) 2010, 207, 793–800. [Google Scholar] [CrossRef]
- Niu, Z.; Li, Y. Removal and Utilization of Capping Agents in Nanocatalysis. Chem. Mater. 2014, 26, 72–83. [Google Scholar] [CrossRef]
- Aliaga, C.; Park, J.Y.; Yamada, Y.; Lee, H.S.; Tsung, C.-K.; Yang, P.; Somorjai, G.A. Sum Frequency Generation and Catalytic Reaction Studies of the Removal of Organic Capping Agents from Pt Nanoparticles by UV–Ozone Treatment. J. Phys. Chem. C 2009, 113, 6150–6155. [Google Scholar] [CrossRef]
- Chen, Y.; Fernandes, A.A.; Erbe, A. Control of shape and surface crystallography of gold nanocrystals for electrochemical applications. Electrochim. Acta 2013, 113, 810–816. [Google Scholar] [CrossRef]
- Grzelczak, M.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L.M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Cargnello, M.; Chen, C.; Diroll, B.T.; Doan-Nguyen, V.V.T.; Gorte, R.J.; Murray, C.B. Efficient Removal of Organic Ligands from Supported Nanocrystals by Fast Thermal Annealing Enables Catalytic Studies on Well-Defined Active Phases. J. Am. Chem. Soc. 2015, 137, 6906–6911. [Google Scholar] [CrossRef]
- Ansar, S.M.; Ameer, F.S.; Hu, W.; Zou, S.; Pittman, C.U.; Zhang, D. Removal of Molecular Adsorbates on Gold Nanoparticles Using Sodium Borohydride in Water. Nano Lett. 2013, 13, 1226–1229. [Google Scholar] [CrossRef]
- Xiao, X.; Pan, S.; Jang, J.S.; Fan, F.-R.F.; Bard, A.J. Single Nanoparticle Electrocatalysis: Effect of Monolayers on Particle and Electrode on Electron Transfer. J. Phys. Chem. C 2009, 113, 14978–14982. [Google Scholar] [CrossRef]
- Horibe, T.; Zhang, J.; Oyama, M. Effects of Capping Reagents on the Electron Transfer Reactions on Gold Nanoparticle-Attached Indium Tin Oxide Electrodes. Electroanalysis 2007, 19, 847–852. [Google Scholar] [CrossRef]
- Nakaso, K.; Han, B.; Ahn, K.H.; Choi, M.; Okuyama, K. Synthesis of non-agglomerated nanoparticles by an electrospray assisted chemical vapor deposition (ES-CVD) method. J. Aerosol Sci. 2003, 34, 869–881. [Google Scholar] [CrossRef]
- Goodman, D.W. Model Studies in Catalysis Using Surface Science Probes. Chem. Rev. 1995, 95, 523–536. [Google Scholar] [CrossRef]
- Gong, J. Structure and Surface Chemistry of Gold-Based Model Catalysts. Chem. Rev. 2012, 112, 2987–3054. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.; Lee, H.; Lee, H.; Lee, M.; Yang, S.; Hwang, A.; Kim, S.-I.; Park, J.Y.; Choo, J.; Kang, T.; et al. Surfactant-Free Vapor-Phase Synthesis of Single-Crystalline Gold Nanoplates for Optimally Bioactive Surfaces. Chem. Mater. 2017, 29, 8747–8756. [Google Scholar] [CrossRef]
- Okumura, M.; Nakamura, S.; Tsubota, S.; Nakamura, T.; Azuma, M.; Haruta, M. Chemical vapor deposition of gold on Al2O3, SiO2, and TiO2 for the oxidation of CO and of H2. Catal. Lett. 1998, 51, 53–58. [Google Scholar] [CrossRef]
- Jen Cho, S.; Suri, A.; Mei, X.; Ouyang, J. In situ deposition of gold nanostructures with well-defined shapes on unfunctionalized reduced graphene oxide through chemical reduction of a dry gold precursor with ethylene glycol vapor. RSC Adv. 2013, 3, 1201–1209. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, X.; Zeng, W.; Zhu, X.; Hu, H.; Duan, H. Vapor-phase preparation of gold nanocrystals by chloroauric acid pyrolysis. J. Colloid Interface Sci. 2015, 439, 21–27. [Google Scholar] [CrossRef]
- Kang, T.; Min Yoo, S.; Kang, M.; Lee, H.; Kim, H.; Lee, S.Y.; Kim, B. Single-step multiplex detection of toxic metal ions by Au nanowires-on-chip sensor using reporter elimination. Lab Chip 2012, 12, 3077–3081. [Google Scholar] [CrossRef]
- Yoo, Y.; Seo, K.; Han, S.; Varadwaj, K.S.K.; Kim, H.Y.; Ryu, J.H.; Lee, H.M.; Ahn, J.P.; Ihee, H.; Kim, B. Steering Epitaxial Alignment of Au, Pd, and AuPd Nanowire Arrays by Atom Flux Change. Nano Lett. 2010, 10, 432–438. [Google Scholar] [CrossRef]
- Makela, M.; Hatanpaa, T.; Mizohata, K.; Raisanen, J.; Ritala, M.; Leskela, M. Thermal Atomic Layer Deposition of Continuous and Highly Conducting Gold Thin Films. Chem. Mater. 2017, 29, 6130–6136. [Google Scholar]
- Makela, M.; Hatanpaa, T.; Ritala, M.; Leskela, M. Potential gold(I) precursors evaluated for atomic layer deposition. J. Vac. Sci. Technol. A 2017, 35, 01B112. [Google Scholar] [CrossRef]
- Griffiths, M.B.; Koponen, S.E.; Mandia, D.J.; McLeod, J.F.; Coyle, J.P.; Sims, J.J.; Giorgi, J.B.; Sirianni, E.R.; Yap, G.P.; Barry, S.T. Surfactant directed growth of gold metal nanoplates by chemical vapor deposition. Chem. Mater. 2015, 27, 6116–6124. [Google Scholar] [CrossRef]
- Gammons, C.H.; Yu, Y.; Williams-Jones, A.E. The disproportionation of gold(I) chloride complexes at 25 to 200 °C. Geochim. Cosmochim. Acta 1997, 61, 1971–1983. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Zhu, X.; Li, Z.; Shang, Z.; Duan, H. Vapor-phase preparation of single-crystalline thin gold microplates using HAuCl4 as the precursor for plasmonic applications. RSC Adv. 2016, 6, 74937–74943. [Google Scholar] [CrossRef]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Lu, X.; Tuan, H.Y.; Korgel, B.A.; Xia, Y. Facile synthesis of gold nanoparticles with narrow size distribution by using AuCl or AuBr as the precursor. Chem. Eur. J. 2008, 14, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Bergamini, G.; Ceroni, P.; Balzani, V.; Gingras, M.; Raimundo, J.-M.; Morandi, V.; Merli, P.G. Synthesis of small gold nanoparticles: Au(i) disproportionation catalyzed by a persulfurated coronene dendrimer. Chem. Commun. 2007, 40, 4167–4169. [Google Scholar] [CrossRef]
- Hee Shin, D.; Min Kim, J.; Wook Jang, C.; Hwan Kim, J.; Kim, S.; Choi, S.-H. Annealing effects on the characteristics of AuCl3-doped graphene. J. App. Phys. 2013, 113, 064305. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, K.K.; Jo, Y.W.; Park, M.H.; Chae, S.J.; Duong, D.L.; Yang, C.W.; Kong, J.; Lee, Y.H. Role of anions in the AuCl3-doping of carbon nanotubes. Acs Nano 2011, 5, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Parkhomenko, R.; Morozova, B.N.; Zharkova, I.G.; Shubin, V.Y.; Trubin, S.; Kriventsov, V.; Kuchumov, M.B.; Koretskaya, P.T.; Igumenov, I. Deposition of Au Thin Films and Nanoparticles by MOCVD. Chem. Vap. Depos. 2012, 18, 336–342. [Google Scholar] [CrossRef]
- Ali Umar, A.; Oyama, M. Formation of Gold Nanoplates on Indium Tin Oxide Surface: Two-dimensional Crystal Growth from Gold Nanoseed Particles in the Presence of Poly (vinylpyrrolidone). Cryst. Growth Des. 2006, 6, 818–821. [Google Scholar] [CrossRef]
- Germain, V.; Li, J.; Ingert, D.; Wang, Z.L.; Pileni, M.P. Stacking Faults in Formation of Silver Nanodisks. J. Phys. Chem. B 2003, 107, 8712–8720. [Google Scholar] [CrossRef]
- Kwon, T.; Jun, M.; Kim, H.Y.; Oh, A.; Park, J.; Baik, H.; Joo, S.H.; Lee, K. Vertex-Reinforced PtCuCo Ternary Nanoframes as Efficient and Stable Electrocatalysts for the Oxygen Reduction Reaction and the Methanol Oxidation Reaction. Adv. Funct. Mater. 2018, 28, 1706440. [Google Scholar] [CrossRef]
- Huang, H.; Yang, S.; Vajtai, R.; Wang, X.; Ajayan, P.M. Pt-decorated 3D architectures built from graphene and graphitic carbon nitride nanosheets as efficient methanol oxidation catalysts. Adv. Mater. 2014, 26, 5160–5165. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, W.; Cai, Q. Well-Dispersed PtAu Nanoparticles Loaded into Anodic Titania Nanotubes: A High Antipoison and Stable Catalyst System for Methanol Oxidation in Alkaline Media. J. Phys. Chem. C 2007, 111, 16613–16617. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.-B.; Wang, Z.-L.; Wang, L.-M.; Xing, W.; Liu, X. One-step and rapid synthesis of “clean” and monodisperse dendritic Pt nanoparticles and their high performance toward methanol oxidation and p-nitrophenol reduction. Nanoscale 2012, 4, 1549–1552. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Liao, H.-Y.; Wu, Y.-F.; Kuo, P.-L. Benzylamine-Assisted Noncovalent Exfoliation of Graphite-Protecting Pt Nanoparticles Applied as Catalyst for Methanol Oxidation. ACS Appl. Mater. Interfaces 2011, 3, 2169–2172. [Google Scholar] [CrossRef]
- Siyu, H.; Ying, C. Electrochemical Catalytic Oxidation Performance of a Nanoporous Gold Electrode. Rare Met. Mater. Eng. 2015, 44, 2156–2158. [Google Scholar] [CrossRef]
- Kita, H.; Ye, S.; Sugimura, K. Effects of adsorbed CO on the electrode reactions at a platinum electrode. J. Electroanal. Chem. Interfacial Electrochem. 1991, 297, 283–296. [Google Scholar] [CrossRef]
- Kwon, Y.; Lai, S.C.; Rodriguez, P.; Koper, M.T. Electrocatalytic oxidation of alcohols on gold in alkaline media: Base or gold catalysis? J. Am. Chem. Soc. 2011, 133, 6914–6917. [Google Scholar] [CrossRef]
- Ketchie, W.C.; Fang, Y.-L.; Wong, M.S.; Murayama, M.; Davis, R.J. Influence of gold particle size on the aqueous-phase oxidation of carbon monoxide and glycerol. J. Catal. 2007, 250, 94–101. [Google Scholar] [CrossRef]
- Zope, B.N.; Hibbitts, D.D.; Neurock, M.; Davis, R.J. Reactivity of the Gold/Water Interface during Selective Oxidation Catalysis. Science 2010, 330, 74. [Google Scholar] [CrossRef] [PubMed]
- Pedireddy, S.; Lee, H.K.; Tjiu, W.W.; Phang, I.Y.; Tan, H.R.; Chua, S.Q.; Troadec, C.; Ling, X.Y. One-step synthesis of zero-dimensional hollow nanoporous gold nanoparticles with enhanced methanol electrooxidation performance. Nat. Commun. 2014, 5, 4947. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, D.; Lakshminarayanan, V. Electrochemically grown mesoporous gold film as high surface area material for electro-oxidation of alcohol in alkaline medium. J. Phys. Chem. C 2009, 113, 14922–14926. [Google Scholar] [CrossRef]
- Trasatti, S.; Petrii, O. Real surface area measurements in electrochemistry. Pure Appl. Chem. 1991, 63, 711–734. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Lou, Y.; Maye, M.M.; Han, L.; Luo, J.; Zhong, C.-J. Gold–platinum alloy nanoparticle assembly as catalyst for methanol electrooxidation. Chem. Commun. 2001, 473–474. [Google Scholar] [CrossRef]
- Jebaraj, A.J.J.; de Godoi, D.R.M.; Scherson, D.A. Pronounced Surface Sensitivity of Hydroxylamine Oxidation on Gold Single-Crystal Electrodes in Acidic and Neutral Aqueous Solutions. Acs Catal. 2012, 2, 911–915. [Google Scholar] [CrossRef]
- Chen, Y.; Schuhmann, W.; Hassel, A.W. Electrocatalysis on gold nanostructures: Is the {1 1 0} facet more active than the {1 1 1} facet? Electrochem. Commun. 2009, 11, 2036–2039. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Park, K.; Kim, B.; Kang, T. Low-Temperature Vapor-Phase Synthesis of Single-Crystalline Gold Nanostructures: Toward Exceptional Electrocatalytic Activity for Methanol Oxidation Reaction. Nanomaterials 2019, 9, 595. https://doi.org/10.3390/nano9040595
Yang S, Park K, Kim B, Kang T. Low-Temperature Vapor-Phase Synthesis of Single-Crystalline Gold Nanostructures: Toward Exceptional Electrocatalytic Activity for Methanol Oxidation Reaction. Nanomaterials. 2019; 9(4):595. https://doi.org/10.3390/nano9040595
Chicago/Turabian StyleYang, Siyeong, Kkotchorong Park, Bongsoo Kim, and Taejoon Kang. 2019. "Low-Temperature Vapor-Phase Synthesis of Single-Crystalline Gold Nanostructures: Toward Exceptional Electrocatalytic Activity for Methanol Oxidation Reaction" Nanomaterials 9, no. 4: 595. https://doi.org/10.3390/nano9040595
APA StyleYang, S., Park, K., Kim, B., & Kang, T. (2019). Low-Temperature Vapor-Phase Synthesis of Single-Crystalline Gold Nanostructures: Toward Exceptional Electrocatalytic Activity for Methanol Oxidation Reaction. Nanomaterials, 9(4), 595. https://doi.org/10.3390/nano9040595