Lipidic Liquid Crystalline Cubic Phases and Magnetocubosomes as Methotrexate Carriers
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Cubic Phases and Cubosomes
2.2.1. Dynamic Light Scattering (DLS) and Zeta Potential
2.2.2. Cryogenic Transmission Electron Microscopy (Cryo-TEM)
2.3. Electrochemical Measurements
2.4. Modeling of the Kinetics of Drug Release
2.5. Magnetic Field Generator
2.6. Spectroscopic Measurements
3. Results and Discussion
3.1. Structural Characterization of the MTX-Doped Cubic Phases
3.2. Electrochemical Measurements
3.2.1. Methotrexate Incorporated in the Monoolein Cubic Phase
3.2.2. Behavior of MTX Incorporated into Hybrid LCP Systems
3.2.3. MTX Incorporated into Magnetocubosomes
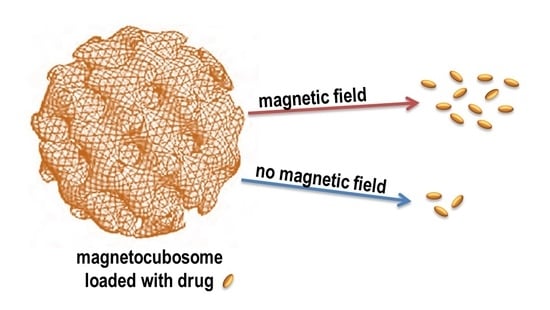
3.3. Low-Frequency Alternating Magnetic Field (AMF)-Stimulated Drug Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MTX | methotrexate |
| SAXS | small angle X-ray scattering |
| LCP | liquid crystalline phase |
| DDS | drug delivery system |
| AMF | alternating magnetic field |
| MO | monoolein |
| MNPs | magnetic nanoparticles |
| DLS | dynamic light scattering |
| Cryo-TEM | cryogenic transmission electron microscopy |
| DPV | differential pulse voltammetry |
| GC | glassy carbon |
| GCE | glassy carbon electrode |
| SWV | square-wave voltammetry |
| CV | cyclic voltammetry |
| EE | entrapment efficiency |
References
- Genestier, L.; Paillot, R.; Quemeneur, L.; Izeradjene, K.; Revillard, J.-P. Mechanisms of action of methotrexate. Immunopharmacology 2000, 47, 247–257. [Google Scholar] [CrossRef]
- Bleyer, W.A. The clinical pharmacology of Methotrexate. Cancer 1978, 41, 36–51. [Google Scholar] [CrossRef]
- Yoon, S.-A.; Choi, J.R.; Kim, J.-O.; Shin, J.-Y.; Zhang, X.H.; Kang, J.-H. Influence of Reduced Folate Carrier and Dihydrofolate Reductase Genes on Methotrexate-Induced Cytotoxicity. Cancer Res. Treat. 2010, 42, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Kanarek, N.; Keys, H.R.; Cantor, J.R.; Lewis, C.A.; Chan, S.H.; Kunchok, T.; Abu-Remaileh, M.; Freinkman, E.; Schweitzer, L.D.; Sabatini, D.M. Histidine catabolism is a major determinant of methotrexate sensitivity. Nature 2018, 559, 632–655. [Google Scholar] [CrossRef] [PubMed]
- Rozensza, L.A.; Radnay, J. The effect of methotrexate on transformation and mitosis of normal human-blood lymphocytes in-vitro. Blood 1974, 43, 401–409. [Google Scholar]
- Bookbinder, S.A.; Espinoza, L.R.; Fenske, N.A.; Germain, B.F.; Vasey, F.B. Methotrexate therapy in the rheumatic disease. Clin. Exp. Rheumatol. 1984, 2, 185–193. [Google Scholar]
- Zeb, A.; Qureshi, O.S.; Kim, H.-S.; Cha, J.-H.; Kim, H.-S.; Kim, J.-K. Improved skin permeation of methotrexate via nanosized ultradeformable liposomes. Int. J. Nanomed. 2016, 11, 3813–3824. [Google Scholar] [CrossRef] [Green Version]
- Srinivas, P.S.; Babu, D.R.S. Formulation and evaluation of parenteral methotrexate nanoliposomes. Int. J. Pharm. Pharm. Sci. 2014, 6, 295–300. [Google Scholar]
- Rosenholm, J.M.; Peuhu, E.; Bate-Eya, L.T.; Eriksson, J.E.; Sahlgren, C.; Linden, M. Cancer-cell-specific induction of apoptosis using mesoporous silica nanoparticles as drug-delivery vectors. Small 2010, 6, 1234–1241. [Google Scholar] [CrossRef]
- de Oliveira Freitas, L.B.; Gonzalez Bravo, I.J.; de Almeida Macedo, W.A.; de Sousa, E.M.B. Mesoporous silica materials functionalized with folic acid: Preparation, characterization and release profile study with methotrexate. J. Sol-Gel Sci. Technol. 2016, 77, 186–204. [Google Scholar] [CrossRef]
- Kakkar, D.; Dumoga, S.; Kumar, R.; Chuttani, K.; Mishra, A.K. PEGylated solid lipid nanoparticles: Design, methotrexate loading and biological evaluation in animal models. Med. Chem. Commun. 2015, 6, 1452–1463. [Google Scholar] [CrossRef]
- Ferreira, M.; Silva, E.; Barreiros, L.; Segundo, M.A.; Costa Lima, S.A.; Reis, S. Methotrexate loaded lipid nanoparticles for topical management of skin-related diseases: Design, characterization and skin permeation potential. Int. J. Pharm. 2016, 512, 14–21. [Google Scholar] [CrossRef]
- Hashada, R.A.; Ishaka, R.A.H.; Geneidia, A.S.; Mansoura, S. Methotrexate loading in chitosan nanoparticles at a novel pH: Response surface modeling, optimization and characterization. Int. J. Biol. Macromol. 2016, 91, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Pretti, T.S.; Souza, M.A.; Santos, H.T.; Nascimento, A.C.G.G.; Santos, F.J.; Fraga, A.F.; Jafelicci, M.J.; Marques, R.F.C. Drug delivery nanotechnology applied to Methotrexate controlled release in human osteosarcona: In vitro test. Available online: http://www.metallum.com.br/7colaob/resumos/trabalhos_completos/08-020.docx (accessed on 15 April 2019).
- Guo, F.; Fan, Z.; Yang, J.; Li, Y.; Wang, Y.; Zhao, H.; Xie, L.; Hou, Z. A comparative evaluation of hydroxycamptothecin drug nanorods with and without methotrexate prodrug functionalization for drug delivery. Nanoscale Res. Lett. 2016, 11, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.D.F.; Pereira, L.G.R.; Barbosa, L.A.D.O.; Fialho, S.L.; Pereira, B.G.; Patricio, P.S.D.O.; Pinto, F.C.H.; Da Silva, G.R. Efficacy of methotrexate-loaded poly(e-caprolactone) implants in Ehrlich solid tumor-bearing mice. Drug Deliv. 2013, 2, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Hamishehkar, H.; Arsalani, N.; Entezami, A.A. A novel dual-responsive core-crosslinked magnetic-gold nanogel for triggered drug release. Mater. Sci. Eng. C 2016, 68, 436–444. [Google Scholar] [CrossRef]
- Lina, L.; Xua, W.; Lianga, H.; Hea, L.; Liua, S.; Lia, Y.; Lia, B.; Chena, Y. Construction of pH-sensitive lysozyme/pectin nanogel for tumor methotrexate delivery. Colloid. Surface. B 2015, 126, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wang, J.; Cao, F.; Lee, R.J.; Zhai, Z. Lyotropic liquid crystal systems in drug deliver. Drug Discov. Today 2010, 1032–1040. [Google Scholar] [CrossRef]
- Angelova, A.; Garamus, V.M.; Angelov, B. Advances in structural design of lipid-based nanoparticle carriers for delivery of macromolecular drugs, phytochemical and anti-tumor agents. Adv. Colloid Interf. Sci. 2017, 249, 331–345. [Google Scholar] [CrossRef]
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The next generation of smart lipid nanoparticles? Angew. Chem. Int. Ed. 2018, 57, 2–23. [Google Scholar] [CrossRef]
- Kulkarni, C.V.; Wachter, W.; Iglesias-Salto, G.; Engelskirchenb, S.; Ahualliac, S. Monoolein: A magic lipid? Phys. Chem. Chem. Phys. 2011, 13, 3004–3021. [Google Scholar] [CrossRef]
- Nazaruk, E.; Szlȩzak, M.; Górecka, E.; Bilewicz, R.; Osornio, Y.M.; Uebelhart, P.; Landau, E.M. Design and Assembly of pH-Sensitive Lipidic Cubic Phase Matrices for Drug Release. Langmuir 2014, 30, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Negrini, R.; Sanchez-Ferrer, A.; Mezzenga, R. Influence of Electrostatic Interactions on the Release of Charged Molecules from Lipid Cubic Phases. Langmuir 2014, 30, 4280–4288. [Google Scholar] [CrossRef]
- Negrini, R.; Fong, W.-K.; Boyd, B.J.; Mezzenga, R. pH-responsive lyotropic liquid crystals and their potential therapeutic role in cancer treatment. Chem. Commun. 2015, 51, 6671–6674. [Google Scholar] [CrossRef] [PubMed]
- Negrini, R.; Mezzenga, R. pH-Responsive Lyotropic Liquid Crystals for Controlled Drug Delivery. Langmuir 2011, 27, 5296–5303. [Google Scholar] [CrossRef]
- Szlezak, M.; Nieciecka, D.; Joniec, A.; Pękała, M.; Gorecka, E.; Emo, M.; Stébé, M.J.; Krysiński, P.; Bilewicz, R. Monoolein cubic phase gels and cubosomes doped with magnetic nanoparticles - hybrid materials for controlled drug release. ACS Appl. Mater. Inter. 2017, 9, 2796–2805. [Google Scholar] [CrossRef]
- Fong, W.-K.; Negrini, R.; Vallooran, J.J.; Mezzenga, R.; Boyd, B.J. Responsive self-assembled nanostructured lipid systems for drug delivery and diagnostics. J. Colloid Interf. Sci. 2016, 484, 320–339. [Google Scholar] [CrossRef]
- Bonini, M.; Berti, D.; Baglioni, P. Nanostructures for magnetically triggered release of drugs and biomolecules. Curr. Opin. Colloid Interface Sci. 2013, 18, 459–467. [Google Scholar] [CrossRef]
- Vallooran, J.J.; Negrini, R.; Mezzenga, R. Controlling anisotropic drug diffusion in lipid-Fe3O4 nanoparticle hybrid mesophases by magnetic alignment. Langmuir 2013, 29, 999–1004. [Google Scholar] [CrossRef]
- Monnier, C.A.; Burnand, D.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Magnetoliposomes: Opportunities and challenges. Eur. J. Nanomed. 2014, 6, 201–215. [Google Scholar] [CrossRef]
- Joniec, A.; Sek, S.; Krysinski, P. Magnetoliposomes as Potential Carriers of Doxorubicin to Tumours. Chem.-Eur. J. 2016, 22, 17715–17724. [Google Scholar] [CrossRef]
- Montis, C.; Castroflorio, B.; Mendozza, M.; Salvatore, A.; Berti, D.; Baglioni, P. Magnetocubosomes for the delivery and controlled release of therapeutics. J. Colloid Interf. Sci. 2015, 449, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.K.; Kim, J.C. Cubic phase magnetic nanoparticles. Mol. Cryst. Liq. Cryst. 2015, 607, 123–134. [Google Scholar] [CrossRef]
- Wang, M.H.; Kim, J.-C. Magnetic field-responsive cubosomes containing magnetite and poly(N-isopropylacrylamide). J. Controlled Release 2013, 172, e139. [Google Scholar] [CrossRef]
- Ivkov, R.; DeNardo, S.J.; Daum, W.; Foreman, A.R.; Goldstein, R.; Nemkov, V.S.; DeNardo, G.L. Application of High Amplitude Alternating Magnetic Fields for Heat Induction of Nanoparticles Localized in Cancer. Clin. Cancer Res. 2005, 11, 7093–7103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Hyde, S.T.; Andersson, S.; Ericsson, B.; Larsson, K. A Cubic Structure Consisting of a Lipid Bilayer Forming an Infinite Periodic Minimum Surface of the Gyroid Type in the Glycerolmonooleat-Water System. Z. Kristallogr. 1984, 168, 213–219. [Google Scholar] [CrossRef]
- Briggs, J.; Chung, H.; Caffrey, M. The Temperature-Composition Phase Diagram and Mesophase Structure Characterization of the Monoolein/Water System. J. Phys. 1996, 6, 723–751. [Google Scholar] [CrossRef]
- Qiu, H.; Caffrey, M. The Phase Diagram of the Monoolein/Water System: Metastability and Equilibrium Aspects. Biomaterials 2000, 21, 223–234. [Google Scholar] [CrossRef]
- Pontinha, A.D.R.; Jorge, S.M.A.; Diculescu, V.C.; Vivan, M.; Oliveira-Brett, A.M. Antineoplasic Drug Methotrexate Redox Mechanism Using a Glassy Carbon Electrode. Electroanalysis 2012, 24, 917–923. [Google Scholar] [CrossRef]
- Gao, L.; Wu, Y.; Liu, J.; Ye, B. Anodic voltammetric behaviors of methotrexate at a glassy carbon electrode and its determination in spiked human urine. J. Electroanal. Chem. 2007, 610, 131–136. [Google Scholar] [CrossRef]
- Acharya, D.P.; Moffat, B.A.; Polyzos, A.; Waddington, L.; Coia, G.; Wright, D.K.; Wang, H.X.; Egan, G.F.; Muir, B.W.; Hartley, P.G. Cubic mesophase nanoparticles doped with superparamagnetic iron oxide nanoparticles: A new class of MRI contrast agent. RSC Adv. 2012, 2, 6655–6662. [Google Scholar] [CrossRef]
- Nazaruk, E.; Miszta, P.; Filipek, S.; Landau, E.M.; Bilewicz, R. Lyotropic cubic phase gels for drug delivery: Tuning diffusion and sustained release from the mesophase. Langmuir 2015, 31, 12753–12761. [Google Scholar] [CrossRef] [PubMed]
- Mendozza, M.; Montis, C.; Caselli, L.; Wolf, M.; Baglionia, P.; Berti, D. On the thermotropic and magnetotropic phase behavior of lipid liquid crystals containing magnetic nanoparticles. Nanoscale 2018, 10, 3480–3488. [Google Scholar] [CrossRef] [PubMed]
- Reimhult, E. Nanoparticle-triggered release from lipid membrane vesicles. New Biotechnol. 2015, 32, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Bixner, O.; Reimhult, E. Controlled megnetosomes: Embedding of magnetic nanoparticles into membranes of monodisperse lipid vesicles. J. Colloid Interf. Sci. 2016, 466, 62–71. [Google Scholar] [CrossRef]
- Shaghasemi, B.S.; Virk, M.M.; Reimhult, E. Optimization of Magneto-thermally Controlled Release Kinetics by Tuning of Magnetoliposome Composition and Structure. Sci. Rep. 2017, 7, 7474. [Google Scholar] [CrossRef] [Green Version]
- Fortin, J.-P.; Gazeau, F.; Wilhelm, C. Intracellular heating of living cells through Néel relaxation of magnetic nanoparticles. Eur. Biophys. J. 2008, 37, 223–228. [Google Scholar] [CrossRef]
- Leesajakul, W.; Nakano, M.; Taniguchi, A.; Handa, A. Interaction of cubosomes with plasma components resulting in the destabilization of cubosomes in plasma. Colloid. Surfaces B 2004, 34, 253–258. [Google Scholar] [CrossRef]
- Tran, N.; Bye, N.; Moffat, B.A.; Wright, D.K.; Cuddihy, A.; Hinton, T.M.; Hawley, A.M.; Reynolds, N.P.; Waddington, L.J.; Mulet, X.; et al. Dual-modality NIRF-MRI cubosomes and hexosomes: High throughput formulation and in vivo biodistribution. Mater. Sci. Eng. C 2017, 71, 584–593. [Google Scholar] [CrossRef]
- Falchi, A.M.; Rosa, A.; Atzeri, A.; Incani, A.; Lampis, S.; Meli, V.; Caltagirone, C.; Murgia, S. Effects of monoolein based cubosome formulations on lipid droplets and mitochondria of HeLa cells. Toxicol. Res. 2015, 4, 1025–1036. [Google Scholar] [CrossRef]
- Biffi, S.; Andolfi, L.; Caltagirone, C.; Garrovo, C.; Falchi, A.M.; Lippolis, V.; Lorenzon, A.; Maco, P.; Meli, V.; Monduzzi, M.; et al. Cubosomes for in vivo fluorescence lifetime imaging. Nanotechnology 2017, 28, 055102. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, E.; Majkowska, A.; Bilewicz, R. Lipidic Cubic-Phase Nanoparticles—Cubosomes for Efficient Drug Delivery to Cancer Cells. ChemPlusChem 2017, 82, 570–575. [Google Scholar] [CrossRef]
- Nazaruk, E.; Majkowska, A.; Godlewska, M.; Salamonczyk, M.; Gawel, D. Electrochemical and biological characterization of lyotropic liquid crystalline phases—Retardation of drug release from hexagonal mesophases. J. Electroanal. Chem. 2018, 813, 208–215. [Google Scholar] [CrossRef]
- Godlewska, M.; Majkowska-Pilip, A.; Stachurska, A.; Gawel, D.; Nazaruk, E. Voltammetric and biological studies of folate-targeted non-lamellar lipid mesophases. Electrochim. Acta 2019, 299, 1–11. [Google Scholar] [CrossRef]








| T [°C] | Symmetry | a [nm] | l [nm] | dw [nm] | |
|---|---|---|---|---|---|
| MO/aq* 60/40% | 25 | Pn3̅m | 10.2 | 1.7 | 4.5 |
| 37 | Pn3̅m | 9.5 | 1.6 | 4.2 | |
| MO/MTX/aq* 59/1/40% | 25 | Pn3̅m | 10.3 | 1.8 | 4.5 |
| 37 | Pn3̅m | 9.8 | 1.7 | 4.3 | |
| MO/MTX/aq* 58/2/40% | 25 | Pn3̅m | 10.3 | 1.8 | 4.6 |
| 37 | Pn3̅m | 9.7 | 1.6 | 4.3 |
| Korsmeyer–Peppas | Higuchi | ||||
|---|---|---|---|---|---|
| % MTX | n | R2 | k [%/hn] | kH [%/h] | R2 |
| 1.0 at 25 °C | 0.44 ± 0.05 | 0.986 ± 0.004 | 56.08 ± 5.40 | 47.49 ± 1.49 | 0.977 ± 0.010 |
| 1.0 at 25 °C CUV-Vis | 0.44 ± 0.01 | 0.998 ± 0.001 | 61.20±1.92 | 58.78 ± 1.32 | 0.997±0.001 |
| 1.0 at 37 °C | 0.65 ± 0.19 | 0.921 ± 0.102 | 66.36 ± 6.32 | a | a |
| 0.5 at 25 °C | 0.41 ± 0.03 | 0.967 ± 0.006 | 62.34 ± 2.22 | 40.51 ± 9.49 | 0.943 ± 0.038 |
| 0.5 at 37 °C | 0.56 ± 0.11 | 0.950 ± 0.043 | 61.38 ± 8.76 | 66.22 ± 4.21 | 0.966 ± 0.020 |
| 0.5 and 2% MNPs at 25 °C | 0.47 ± 0.06 | 0.949 ± 0.014 | 60.60 ± 2.73 | 62.68 ± 5.85 | 0.945 ± 0.021 |
| 0.25 at 25 °C | 0.45 ± 0.08 | 0.975 ± 0.005 | 57.12 ± 3.80 | 53.97 ± 3.38 | 0.982 ± 0.008 |
| 0.25 at 37 °C | 0.57 ± 0.09 | 0.943 ± 0.014 | 63.51 ± 4.18 | 72.21 ± 4.11 | 0.962 ± 0.009 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mierzwa, M.; Cytryniak, A.; Krysiński, P.; Bilewicz, R. Lipidic Liquid Crystalline Cubic Phases and Magnetocubosomes as Methotrexate Carriers. Nanomaterials 2019, 9, 636. https://doi.org/10.3390/nano9040636
Mierzwa M, Cytryniak A, Krysiński P, Bilewicz R. Lipidic Liquid Crystalline Cubic Phases and Magnetocubosomes as Methotrexate Carriers. Nanomaterials. 2019; 9(4):636. https://doi.org/10.3390/nano9040636
Chicago/Turabian StyleMierzwa, Monika, Adrianna Cytryniak, Paweł Krysiński, and Renata Bilewicz. 2019. "Lipidic Liquid Crystalline Cubic Phases and Magnetocubosomes as Methotrexate Carriers" Nanomaterials 9, no. 4: 636. https://doi.org/10.3390/nano9040636
APA StyleMierzwa, M., Cytryniak, A., Krysiński, P., & Bilewicz, R. (2019). Lipidic Liquid Crystalline Cubic Phases and Magnetocubosomes as Methotrexate Carriers. Nanomaterials, 9(4), 636. https://doi.org/10.3390/nano9040636