Combined Influence of Meso- and Macroporosity of Soft-Hard Templated Carbon Electrodes on the Performance of Li-O2 Cells with Different Configurations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mesoporous Carbon Preparation
2.2. Meso-Macroporous Carbon Preparation
2.3. Porous Carbon Characterization
2.4. Electrode Preparation and Electrochemical Tests
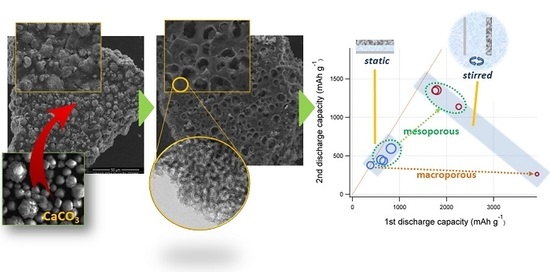
3. Results and Discussion
3.1. Porous Carbon Characterization
3.2. Electrochemical Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mirzaeian, M.; Hall, P.J. Preparation of controlled porosity carbon aerogels for energy storage in rechargeable lithium oxygen batteries. Electrochim. Acta 2009, 54, 7444–7451. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.B.; Lee, D.J.; Roev, V.; Im, D.; Doo, S.G. Effect of porosity on electrochemical properties of carbon materials as cathode for lithium-oxygen battery. J. Power Sources 2013, 244, 494–498. [Google Scholar] [CrossRef]
- Park, J.; Jeong, J.; Lee, S.; Jo, C.; Lee, J. Effect of mesoporous structured cathode materials on charging potentials and rate capability of lithium–oxygen batteries. ChemSusChem 2015, 3146–3152. [Google Scholar] [CrossRef] [PubMed]
- Younesi, S.R.; Urbonaite, S.; Björefors, F.; Edström, K. Influence of the cathode porosity on the discharge performance of the lithium-oxygen battery. J. Power Sources 2011, 196, 9835–9838. [Google Scholar] [CrossRef]
- Nimon, V.Y.; Visco, S.J.; De Jonghe, L.C.; Volfkovich, Y.M.; Bograchev, D.A. Modeling and experimental study of porous carbon cathodes in Li-O2 cells with non-aqueous electrolyte. ECS Electrochem. Lett. 2013, 2, A33–A35. [Google Scholar] [CrossRef]
- Kuboki, T.; Okuyama, T.; Ohsaki, T.; Takami, N. Lithium-air batteries using hydrophobic room temperature ionic liquid electrolyte. J. Power Sources 2005, 146, 766–769. [Google Scholar] [CrossRef]
- Tran, C.; Yang, X.Q.; Qu, D. Investigation of the gas-diffusion-electrode used as lithium/air cathode in non-aqueous electrolyte and the importance of carbon material porosity. J. Power Sources 2010, 195, 2057–2063. [Google Scholar]
- Ding, N.; Chien, S.; Hor, T.S.A.; Lum, R.; Zong, Y.; Liu, Z. Influence of carbon pore size on the discharge capacity of Li-O2 batteries. J. Mater. Chem. A 2014, 2, 12433–12441. [Google Scholar] [CrossRef]
- Yang, X.H.; He, P.; Xia, Y.Y. Preparation of mesocellular carbon foam and its application for lithium/oxygen battery. Electrochem. Commun. 2009, 11, 1127–1130. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Li, J.; Wang, M.; Nie, H.; Zhang, F. The use of mixed carbon materials with improved oxygen transport in a lithium-air battery. J. Power Sources 2013, 240, 390–396. [Google Scholar] [CrossRef]
- Aklalouch, M.; Olivares-Marín, M.; Lee, R.-C.; Palomino, P.; Enciso, E.; Tonti, D. Mass-transport Control on the Discharge Mechanism in Li–O2 Batteries Using Carbon Cathodes with Varied Porosity. ChemSusChem 2015, 8, 3465–3471. [Google Scholar] [CrossRef]
- Read, J. Characterization of the lithium/oxygen organic electrolyte battery. J. Electrochem. Soc. 2002, 149, A1190–A1195. [Google Scholar] [CrossRef]
- Horstmann, B.; Gallant, B.; Mitchell, R.; Bessler, W.G.; Shao-Horn, Y.; Bazant, M.Z. Rate-dependent morphology of Li2O2 growth in Li-O2 batteries. J. Phys. Chem. Lett. 2013, 4, 4217–4222. [Google Scholar] [CrossRef]
- Mitchell, R.R.; Gallant, B.M.; Shao-Horn, Y.; Thompson, C.V. Mechanisms of morphological evolution of Li2O2 particles during electrochemical growth. J. Phys. Chem. Lett. 2013, 4, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.D.; Radtke, C.; Black, R.; Trudeau, M.L.; Zaghib, K.; Nazar, L.F. Current density dependence of peroxide formation in the Li-O2 battery and its effect on charge. Energy Environ. Sci. 2013, 6, 1772–1778. [Google Scholar] [CrossRef]
- Xue, K.H.; McTurk, E.; Johnson, L.; Bruce, P.G.; Franco, A.A. A comprehensive model for non-aqueous lithium air batteries involving different reaction mechanisms. J. Electrochem. Soc. 2015, 162, A614–A621. [Google Scholar] [CrossRef]
- Liu, Y.; Suo, L.; Lin, H.; Yang, W.; Fang, Y.; Liu, X.; Wang, D.; Hu, Y.S.; Han, W.; Chen, L. Novel approach for a high-energy-density Li-air battery: Tri-dimensional growth of Li2O2 crystals tailored by electrolyte Li + ion concentrations. J. Mater. Chem. A 2014, 2, 9020–9024. [Google Scholar] [CrossRef]
- Johnson, L.; Li, C.; Liu, Z.; Chen, Y.; Freunberger, S.A.; Ashok, P.C.; Praveen, B.B.; Dholakia, K.; Tarascon, J.M.; Bruce, P.G. The role of LiO2 solubility in O2 reduction in aprotic solvents and its consequences for Li-O2 batteries. Nat. Chem. 2014, 6, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Aetukuri, N.B.; McCloskey, B.D.; Garcia, J.M.; Krupp, L.E.; Viswanathan, V.; Luntz, A.C. Solvating additives drive solution-mediated electrochemistry and enhance toroid growth in non-aqueous Li-O2 batteries. Nat. Chem. 2015, 7, 50–56. [Google Scholar] [CrossRef]
- Cecchetto, L.; Tesio, A.Y.; Olivares-Marin, M.; Espinasa, M.G.; Croce, F.; Tonti, D. Tailoring oxygen redox reactions in ionic liquid based Li/O2 batteries by means of the Li+ dopant concentration. Sustainable Energy Fuels 2018, 2, 118–124. [Google Scholar] [CrossRef]
- Olivares-Marín, M.; Palomino, P.; Enciso, E.; Tonti, D. Simple method to relate experimental pore size distribution and discharge capacity in cathodes for Li/O2 batteries. J. Phys. Chem. C 2014, 118, 20772–20783. [Google Scholar]
- Li, J.; Zhang, H.; Zhang, Y.; Wang, M.; Zhang, F.; Nie, H. A hierarchical porous electrode using a micron-sized honeycomb-like carbon material for high capacity lithium-oxygen batteries. Nanoscale 2013, 5, 4647–4651. [Google Scholar] [CrossRef] [PubMed]
- Chervin, C.N.; Wattendorf, M.J.; Long, J.W.; Kucko, N.W.; Rolison, D.R. Carbon nanofoam-based cathodes for Li-O2 batteries: Correlation of pore-solid architecture and electrochemical performance. J. Electrochem. Soc. 2013, 160, A1510–A1516. [Google Scholar] [CrossRef]
- Gaya, C.; Yin, Y.; Torayev, A.; Mammeri, Y.; Franco, A.A. Investigation of bi-porous electrodes for lithium oxygen batteries. Electrochim. Acta 2018, 279, 118–127. [Google Scholar] [CrossRef]
- Elia, G.A.; Hassoun, J.; Kwak, W.J.; Sun, Y.K.; Scrosati, B.; Mueller, F.; Bresser, D.; Passerini, S.; Oberhumer, P.; Tsiouvaras, N.; et al. An advanced lithium-air battery exploiting an ionic liquid-based electrolyte. Nano Lett. 2014, 14, 6572–6577. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Marín, M.; Sorrentino, A.; Pereiro, E.; Tonti, D. Discharge products of ionic liquid-based Li-O2 batteries observed by energy dependent soft x-ray transmission microscopy. J. Power Sources 2017, 359, 234–241. [Google Scholar] [CrossRef]
- Lazzari, M.; Arbizzani, C.; Soavi, F.; Mastragostino, M. EDLCs based on solvent-free ionic liquids. In Supercapacitors: Materials, Systems, and Applications; Béguin, F., Frąckowiak, E., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 289–306. [Google Scholar]
- Mastragostino, M.; Soavi, F. CAPACITORS|Electrochemical capacitors: Ionic liquid electrolytes. In Encyclopedia of Electrochemical Power Sources; Garche, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 649–657. [Google Scholar]
- Fernicola, A.; Croce, F.; Scrosati, B.; Watanabe, T.; Ohno, H. LiTFSI-BEPyTFSI as an improved ionic liquid electrolyte for rechargeable lithium batteries. J. Power Sources 2007, 174, 342–348. [Google Scholar] [CrossRef]
- Shin, J.H.; Cairns, E.J. N-Methyl-(n-butyl)pyrrolidinium bis(trifluoromethanesulfonyl)imide-LiTFSI–poly(ethylene glycol) dimethyl ether mixture as a Li/S cell electrolyte. J. Power Sources 2008, 177, 537–545. [Google Scholar] [CrossRef]
- Kar, M.; Simons, T.J.; Forsyth, M.; Macfarlane, D.R. Ionic liquid electrolytes as a platform for rechargeable metal-air batteries: A perspective. Phys. Chem. Chem. Phys. 2014, 16, 18658–18674. [Google Scholar] [CrossRef]
- Herranz, J.; Garsuch, A.; Gasteiger, H.A. Using rotating ring disc electrode voltammetry to quantify the superoxide radical stability of aprotic Li-air battery electrolytes. J. Phys. Chem. C 2012, 116, 19084–19094. [Google Scholar] [CrossRef]
- Cecchetto, L.; Salomon, M.; Scrosati, B.; Croce, F. Study of a Li-air battery having an electrolyte solution formed by a mixture of an ether-based aprotic solvent and an ionic liquid. J. Power Sources 2012, 213, 233–238. [Google Scholar] [CrossRef]
- Allen, C.J.; Mukerjee, S.; Plichta, E.J.; Hendrickson, M.A.; Abraham, K.M. Oxygen electrode rechargeability in an ionic liquid for the li-air battery. J. Phys. Chem. Lett. 2011, 2, 2420–2424. [Google Scholar] [CrossRef]
- Górka, J.; Jaroniec, M. Influence of temperature, carbon precursor/copolymer ratio and acid concentration on adsorption and structural properties of mesoporous carbons prepared by soft-templating. Colloids Surf. Physicochem. Eng. Aspects 2009, 352, 113–117. [Google Scholar]
- Zhai, Y.; Dou, Y.; Liu, X.; Park, S.S.; Ha, C.S.; Zhao, D. Soft-template synthesis of ordered mesoporous carbon/nanoparticle nickel composites with a high surface area. Carbon 2011, 49, 545–555. [Google Scholar] [CrossRef]
- Hwang, D.; Cho, K.; Choi, M.; Yu, Y.; Lee, S.; Ahn, J.; Lim, G.; Han, C.; Lee, J. Effects of sodium dodecyl benzenesulfonic acid (SDBS) on the morphology and the crystal phase of CaCO3. Korean J. Chem. Eng. 2011, 28, 1927–1935. [Google Scholar] [CrossRef]
- Faatz, M.; Gröhn, F.; Wegner, G. Amorphous calcium carbonate: Synthesis and potential intermediate in biomineralization. Adv. Mater. 2004, 16, 996–1000. [Google Scholar] [CrossRef]
- Cai, A.; Xu, X.; Pan, H.; Tao, J.; Liu, R.; Tang, R.; Cho, K. Direct synthesis of hollow vaterite nanospheres from amorphous calcium carbonate nanoparticles via phase transformation. J. Phys. Chem. C 2008, 112, 11324–11330. [Google Scholar] [CrossRef]
- Olivares-Marín, M.; Palomino, P.; Amarilla, J.M.; Enciso, E.; Tonti, D. Effects of architecture on the electrochemistry of binder-free inverse opal carbons as Li-air cathodes in an ionic liquid-based electrolyte. J. Mater. Chem. A 2013, 1, 14270–14279. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Christensen, R.; Hummelshøj, J.S.; Hansen, H.A.; Vegge, T. Reducing systematic errors in oxide species with density functional theory calculations. J. Phys. Chem. C 2015, 119, 17596–17601. [Google Scholar] [CrossRef]
- Xue, K.H.; Nguyen, T.K.; Franco, A.A. Impact of the cathode microstructure on the discharge performance of lithium air batteries: A multiscale model. J. Electrochem. Soc. 2014, 161, E3028–E3035. [Google Scholar] [CrossRef]
- Hou, J.; Yang, M.; Ellis, M.W.; Moore, R.B.; Yi, B. Lithium oxides precipitation in nonaqueous Li-air batteries. Phys. Chem. Chem. Phys. 2012, 14, 13487–13501. [Google Scholar] [CrossRef]
- Zeng, J.; Nair, J.R.; Francia, C.; Bodoardo, S.; Penazzi, N. Aprotic Li-O2 cells: Gas diffusion layer (GDL) as catalyst free cathode and tetraglyme/LiClO4 as electrolyte. Solid State Ion. 2013, 262, 160–164. [Google Scholar] [CrossRef]
- Monaco, S.; Soavi, F.; Mastragostino, M. Role of oxygen mass transport in rechargeable Li/O2 batteries operating with ionic liquids. J. Phys. Chem. Lett. 2013, 4, 1379–1382. [Google Scholar] [CrossRef] [PubMed]
- Soavi, F.; Monaco, S.; Mastragostino, M. Catalyst-free porous carbon cathode and ionic liquid for high efficiency, rechargeable Li/O2 battery. J. Power Sources 2013, 224, 115–119. [Google Scholar] [CrossRef]
- Lim, H.D.; Park, K.Y.; Song, H.; Jang, E.Y.; Gwon, H.; Kim, J.; Kim, Y.H.; Lima, M.D.; Robles, R.O.; Leprõ, X.; et al. Enhanced power and rechargeability of a Li-O2 battery based on a hierarchical-fibril CNT electrode. Adv. Mater. 2013, 25, 1348–1352. [Google Scholar] [CrossRef]








| Sample | SBET (m2 g−1) | Vmicro (cm3 g−1) | Sext (m2 g−1) | Dmax (nm) | Vtotal (cm3 g−1) | Vmeso (cm3 g−1) | %Vmeso (%) |
|---|---|---|---|---|---|---|---|
| MC | 685 | 0.11 | 429 | 11 | 0.64 | 0.53 | 83 |
| MMC-1 | 225 | 0.03 | 163 | 72 | 0.53 | 0.50 | 94 |
| MMC-2 | 303 | 0.04 | 224 | 41 | 0.63 | 0.59 | 94 |
| Super P | 67 | - | 70 | 40 | 0.14 | 0.14 | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivares-Marín, M.; Aklalouch, M.; Tonti, D. Combined Influence of Meso- and Macroporosity of Soft-Hard Templated Carbon Electrodes on the Performance of Li-O2 Cells with Different Configurations. Nanomaterials 2019, 9, 810. https://doi.org/10.3390/nano9060810
Olivares-Marín M, Aklalouch M, Tonti D. Combined Influence of Meso- and Macroporosity of Soft-Hard Templated Carbon Electrodes on the Performance of Li-O2 Cells with Different Configurations. Nanomaterials. 2019; 9(6):810. https://doi.org/10.3390/nano9060810
Chicago/Turabian StyleOlivares-Marín, Mara, Mohamed Aklalouch, and Dino Tonti. 2019. "Combined Influence of Meso- and Macroporosity of Soft-Hard Templated Carbon Electrodes on the Performance of Li-O2 Cells with Different Configurations" Nanomaterials 9, no. 6: 810. https://doi.org/10.3390/nano9060810
APA StyleOlivares-Marín, M., Aklalouch, M., & Tonti, D. (2019). Combined Influence of Meso- and Macroporosity of Soft-Hard Templated Carbon Electrodes on the Performance of Li-O2 Cells with Different Configurations. Nanomaterials, 9(6), 810. https://doi.org/10.3390/nano9060810