A Comparison Study of Functional Groups (Amine vs. Thiol) for Immobilizing AuNPs on Zeolite Surface
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Chemicals
2.2. Preparation of AuNPs
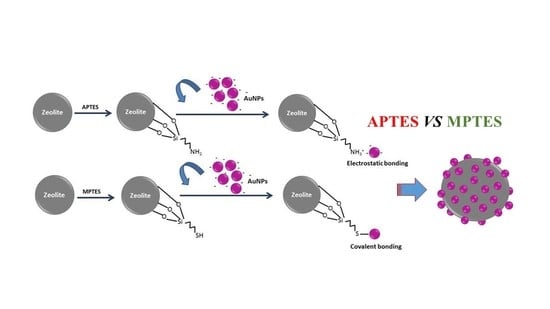
2.3. Salinization of the Surface of the Y Zeolite
2.4. Immobilization of the AuNPs on Silane Modified Zeolite
2.5. Characterizations
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Haruta, M.; Tsubota, S.; Kobayashi, T.; Kageyama, H.; Genet, M.J.; Delmon, B. Low-Temperature Oxidation of CO over Gold Supported on TiO2, α-Fe2O3, and Co3O4. J. Catal. 1993, 144, 175–192. [Google Scholar] [CrossRef]
- Meyer, R.; Lemire, C.; Shaikhutdinov, S.K.; Freund, H.J. Surface chemistry of catalysis by gold. Gold Bull 2004, 37, 72–124. [Google Scholar] [CrossRef] [Green Version]
- Hallett-Tapley, G.L.; Silvero, M.J.; González-Béjar, M.; Grenier, M.; Netto-Ferreira, J.C.; Scaiano, J.C. Plasmon-Mediated Catalytic Oxidation of sec-Phenethyl and Benzyl Alcohols. J. Phys. Chem. C 2011, 115, 10784–10790. [Google Scholar] [CrossRef]
- Bond, G.; Thompson, D. Gold-catalysed oxidation of carbon monoxide. Gold Bull 2000, 33, 41–50. [Google Scholar] [CrossRef]
- Claus, P. Heterogeneously catalysed hydrogenation using gold catalysts. Appl. Catal. A: Gen. 2005, 291, 222–229. [Google Scholar] [CrossRef]
- Aslan, K.; Luhrs, C.C.; Pérez-Luna, V.H. Controlled and Reversible Aggregation of Biotinylated Gold Nanoparticles with Streptavidin. J. Phys. Chem. B 2004, 108, 15631–15639. [Google Scholar] [CrossRef]
- Comotti, M.; Della Pina, C.; Matarrese, R.; Rossi, M. The Catalytic Activity of “Naked” Gold Particles. Angew. Chem. Int. Ed. 2004, 43, 5812–5815. [Google Scholar] [CrossRef]
- Ma, Z.; Dai, S. Development of novel supported gold catalysts: A materials perspective. Nano Res. 2011, 4, 3–32. [Google Scholar] [CrossRef]
- Ishida, T.; Kinoshita, N.; Okatsu, H.; Akita, T.; Takei, T.; Haruta, M. Influence of the Support and the Size of Gold Clusters on Catalytic Activity for Glucose Oxidation. Angew. Chem. 2008, 120, 9405–9408. [Google Scholar] [CrossRef]
- Naya, S.-i.; Teranishi, M.; Kimura, K.; Tada, H. A strong support-effect on the catalytic activity of gold nanoparticles for hydrogen peroxide decomposition. Chem. Commun. 2011, 47, 3230–3232. [Google Scholar] [CrossRef]
- Bokhimi, X.; Zanella, R.; Morales, A. Au/Rutile Catalysts: Effect of Support Dimensions on the Gold Crystallite Size and the Catalytic Activity for CO Oxidation. J. Phys. Chem. C 2007, 111, 15210–15216. [Google Scholar] [CrossRef]
- Soares, J.M.C.; Morrall, P.; Crossley, A.; Harris, P.; Bowker, M. Catalytic and noncatalytic CO oxidation on Au/TiO2 catalysts. J. Catal. 2003, 219, 17–24. [Google Scholar] [CrossRef]
- Minicò, S.; Scirè, S.; Crisafulli, C.; Maggiore, R.; Galvagno, S. Catalytic combustion of volatile organic compounds on gold/iron oxide catalysts. Appl. Catal. B: Environ. 2000, 28, 245–251. [Google Scholar] [CrossRef]
- Guczi, L.; Beck, A.; Frey, K. Role of promoting oxide morphology dictating the activity of Au/SiO2 catalyst in CO oxidation. Gold Bull 2009, 42, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Gu, H.; Wang, J.; Ji, Y.; Wang, Z.; Chen, W.; Xue, G. Facile and controllable fabrication of gold nanoparticles-immobilized hollow silica particles and their high catalytic activity. J. Mater. Chem. A 2013, 1, 12471–12477. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Yuranov, I.; Suvorova, E.I.; Buffat, P.A.; Kiwi-Minsker, L. Highly dispersed gold on activated carbon fibers for low-temperature CO oxidation. J. Catal. 2004, 224, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Larsen, S.C. Nanocrystalline Zeolites and Zeolite Structures: Synthesis, Characterization, and Applications. J. Phys. Chem. C 2007, 111, 18464–18474. [Google Scholar] [CrossRef]
- Lu, J.; Aydin, C.; Browning, N.D.; Gates, B.C. Imaging Isolated Gold Atom Catalytic Sites in Zeolite NaY. Angew. Chem. 2012, 124, 5944–5948. [Google Scholar] [CrossRef]
- Okumura, K.; Yoshino, K.; Kato, K.; Niwa, M. Quick XAFS Studies on the Y-Type Zeolite Supported Au Catalysts for CO−O2 Reaction. J. Phys. Chem. B 2005, 109, 12380–12386. [Google Scholar] [CrossRef]
- Chen, H.; Dai, Y.; Jia, X.; Yu, H.; Yang, Y. Highly selective gas-phase oxidation of ethanol to ethyl acetate over bi-functional Pd/zeolite catalysts. Green Chem. 2016, 18, 3048–3056. [Google Scholar] [CrossRef]
- Chen, H.; Jia, X.; Li, Y.; Liu, C.; Yang, Y. Controlled surface properties of Au/ZSM5 catalysts and their effects in the selective oxidation of ethanol. Catal. Today 2015, 256, 153–160. [Google Scholar] [CrossRef]
- Kubička, D.; Kumar, N.; Venäläinen, T.; Karhu, H.; Kubičková, I.; Österholm, H.; Murzin, D.Y. Metal−Support Interactions in Zeolite-Supported Noble Metals: Influence of Metal Crystallites on the Support Acidity. J. Phys. Chem. B 2006, 110, 4937–4946. [Google Scholar] [CrossRef]
- Petrova, G.P.; Vayssilov, G.N.; Rösch, N. Density functional modeling of reverse hydrogen spillover on zeolite-supported tetrairidium clusters. Chem. Phys. Lett. 2007, 444, 215–219. [Google Scholar] [CrossRef]
- Zhang, X.; Ke, X.; Zhu, H. Zeolite-Supported Gold Nanoparticles for Selective Photooxidation of Aromatic Alcohols under Visible-Light Irradiation. Chem. – A Eur. J. 2012, 18, 8048–8056. [Google Scholar] [CrossRef]
- Valdés, H.; Tardón, R.F.; Zaror, C.A. Role of surface hydroxyl groups of acid-treated natural zeolite on the heterogeneous catalytic ozonation of methylene blue contaminated waters. Chem. Eng. J. 2012, 211–212, 388–395. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Bautista-Toledo, M.I.; Méndez-Díaz, J.D. Enhanced oxidation of sodium dodecylbenzenesulfonate aqueous solution using ozonation catalyzed by base treated zeolite. Chem. Eng. J. 2012, 180, 204–209. [Google Scholar] [CrossRef]
- Kwong, C.W.; Chao, C.Y.H.; Hui, K.S.; Wan, M.P. Catalytic Ozonation of Toluene Using Zeolite and MCM-41 Materials. Environ. Sci. Technol. 2008, 42, 8504–8509. [Google Scholar] [CrossRef]
- Ben Haddada, M.; Blanchard, J.; Casale, S.; Krafft, J.-M.; Vallée, A.; Méthivier, C.; Boujday, S. Optimizing the immobilization of gold nanoparticles on functionalized silicon surfaces: amine- vs thiol-terminated silane. Gold Bull 2013, 46, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Ftouni, J.; Penhoat, M.; Girardon, J.-S.; Addad, A.; Payen, E.; Rolando, C. Immobilization of gold nanoparticles on fused silica capillary surface for the development of catalytic microreactors. Chem. Eng. J. 2013, 227, 103–110. [Google Scholar] [CrossRef]
- Mukhopadhyay, K.; Phadtare, S.; Vinod, V.P.; Kumar, A.; Rao, M.; Chaudhari, R.V.; Sastry, M. Gold Nanoparticles Assembled on Amine-Functionalized Na−Y Zeolite: A Biocompatible Surface for Enzyme Immobilization. Langmuir 2003, 19, 3858–3863. [Google Scholar] [CrossRef]
- Xia, H.B.; Bai, S.O.; Hartmann, J.; Wang, D.Y. Synthesis of Monodisperse Quasi-Spherical Gold Nanoparticles in Water via Silver(I)-Assisted Citrate Reduction. Langmuir 2010, 26, 3585–3589. [Google Scholar] [CrossRef]
- Asadollahi, M.; Bastani, D.; Kazemian, H. Permeation of single gases through TEG liquid membranes modified by Na-Y nano-zeolite particles. Sep. Purif. Technol. 2010, 76, 120–125. [Google Scholar] [CrossRef]
- Song, W.; Li, G.; Grassian, V.H.; Larsen, S.C. Development of Improved Materials for Environmental Applications: Nanocrystalline NaY Zeolites. Environ. Sci. Technol. 2005, 39, 1214–1220. [Google Scholar] [CrossRef]
- Kuge, K.i.; Calzaferri, G. Gold-loaded zeolite A. Microporous Mesoporous Mater. 2003, 66, 15–20. [Google Scholar] [CrossRef]
- Rao, X.; Guyon, C.; Ognier, S.; Silva, B.D.; Chu, C.; Tatoulian, M.; Hassan, A.A. High density gold nanoparticles immobilized on surface via plasma deposited APTES film for decomposing organic compounds in microchannels. Appl. Surf. Sci. 2018, 439. [Google Scholar] [CrossRef]
- Qin, M.; Hou, S.; Wang, L.; Feng, X.; Wang, R.; Yang, Y.; Wang, C.; Yu, L.; Shao, B.; Qiao, M. Two methods for glass surface modification and their application in protein immobilization. Colloids Surf. B: Biointerfaces 2007, 60, 243–249. [Google Scholar] [CrossRef]
- Kang, H.; Kim, M.; Park, K.H. Effective immobilization of gold nanoparticles on core–shell thiol-functionalized GO coated TiO2 and their catalytic application in the reduction of 4-nitrophenol. Appl. Catal. A: Gen. 2015, 502, 239–245. [Google Scholar] [CrossRef]
- Zheng, J.; Zhu, Z.; Chen, H.; Liu, Z. Nanopatterned Assembling of Colloidal Gold Nanoparticles on Silicon. Langmuir 2000, 16, 4409–4412. [Google Scholar] [CrossRef]
- Lin, J.-N.; Chen, J.-H.; Hsiao, C.-Y.; Kang, Y.-M.; Wan, B.-Z. Gold supported on surface acidity modified Y-type and iron/Y-type zeolite for CO oxidation. Appl. Catal. B: Environ. 2002, 36, 19–29. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Q.; Wei, H.; Wang, J.-Q.; Cho, M.; Cho, H.S.; Terasaki, O.; Wan, Y. Aggregation-Free Gold Nanoparticles in Ordered Mesoporous Carbons: Toward Highly Active and Stable Heterogeneous Catalysts. J. Am. Chem. Soc. 2013, 135, 11849–11860. [Google Scholar] [CrossRef]
- Fu, P.; Zhang, P. Uniform dispersion of Au nanoparticles on TiO2 film via electrostatic self-assembly for photocatalytic degradation of bisphenol A. Appl. Catal. B: Environ. 2010, 96, 176–184. [Google Scholar] [CrossRef]
- Dumitriu, E.; Hulea, V.; Kaliaguine, S.; Huang, M.M. Transalkylation of the alkylaromatic hydrocarbons in the presence of ultrastable Y zeolites transalkylation of toluene with trimethylbenzenes. Appl. Catal. A Gen. 1996, 135, 57–81. [Google Scholar] [CrossRef]
- Lecoq, E.; Duday, D.; Bulou, S.; Frache, G.; Hilt, F.; Maurau, R.; Choquet, P. Plasma Polymerization of APTES to Elaborate Nitrogen Containing Organosilicon Thin Films: Influence of Process Parameters and Discussion About the Growing Mechanisms. Plasma Process. Polym. 2013, 10, 250–261. [Google Scholar] [CrossRef]
- Kim, S.S.; Park, J.E.; Lee, J. Properties and antimicrobial efficacy of cellulose fiber coated with silver nanoparticles and 3-mercaptopropyltrimethoxysilane (3-MPTMS). J. Appl. Polym. Sci. 2011, 119, 2261–2267. [Google Scholar] [CrossRef]
- Yang, S.-R.; Kolbesen, B.O. A comparative study of the growth of octadecyltrichlorosilane and 3-mercaptopropyltrimethoxysilane self-assembled monolayers on hydrophilic silicon surfaces. Appl. Surf. Sci. 2008, 255, 1726–1735. [Google Scholar] [CrossRef]
- Sinapi, F.; Forget, L.; Delhalle, J.; Mekhalif, Z. Self-assembly of (3-mercaptopropyl)trimethoxysilane on polycrystalline zinc substrates towards corrosion protection. Appl. Surf. Sci. 2003, 212–213, 464–471. [Google Scholar] [CrossRef]
- Singh, J.; Whitten, J.E. Adsorption of 3-Mercaptopropyltrimethoxysilane on Silicon Oxide Surfaces and Adsorbate Interaction with Thermally Deposited Gold. J. Phys. Chem. C 2008, 112, 19088–19096. [Google Scholar] [CrossRef]
- Wang, Y.P.; Yuan, K.; Li, Q.L.; Wang, L.P.; Gu, S.J.; Pei, X.W. Preparation and characterization of poly(N-isopropylacrylamide) films on a modified glass surface via surface initiated redox polymerization. Mater Lett 5:1736-1740. Mater. Lett. 2005, 59, 1736–1740. [Google Scholar] [CrossRef]
- Lung, C.Y.K.; Heinonen, M.; Kukk, E.; Matinlinna, J.P. Surface modification of titanium with thermally treated polydimethylsiloxane coating and the effect on resin to titanium adhesion. Surf. Interface Anal. 2015, 47, 105–112. [Google Scholar] [CrossRef]
- Briand, E.; Humblot, V.; Landoulsi, J.; Petronis, S.; Pradier, C.-M.; Kasemo, B.; Svedhem, S. Chemical Modifications of Au/SiO2 Template Substrates for Patterned Biofunctional Surfaces. Langmuir Acs J. Surf. Colloids 2011, 27, 678. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, R.; Mu, Y.; Sun, C.; Ji, C.; Zhang, Y.; An, K.; Jia, X.; Zhang, Y. Amino- and Thiol- Polysilsesquioxane Simultaneously Coating on Poly(p-Phenylenetherephthal Amide) Fibers: Bifunctional Adsorbents for Hg(II). Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Faceto, B.; Teixeira-Neto, E.; Pastore, H.O.; Oliveira, C.L.P.; Teixeira-Neto, A.A. On the formation and accessibility of gold nanoparticles confined in SBA-15 mesoporous molecular sieve. Microporous Mesoporous Mater. 2015, 210, 86–93. [Google Scholar] [CrossRef]
- Mashhadizadeh, M.H.; Talemi, R.P. A novel optical DNA biosensor for detection of trace amounts of mercuric ions using gold nanoparticles introduced onto modified glass surface. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 403–409. [Google Scholar] [CrossRef]
- Smith, E.A.; Chen, W. How To Prevent the Loss of Surface Functionality Derived from Aminosilanes. Langmuir Acs J. Surf. Colloids 2008, 24, 12405. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, T.; Hu, R.; Liu, Z. Evaporation-induced self-assembly of gold nanoparticles into a highly organized two-dimensional array. PCCP 2002, 4, 6059. [Google Scholar] [CrossRef]
- Bhat, R.R.; Genzer, J.; Chaney, B.N.; Sugg, H.W.; Liebmann-vinson, A. Controlling the assembly of nanoparticles using surface grafted molecular and macromolecular gradients. Nanotechnology 2003, 14, 1145–1152. [Google Scholar] [CrossRef] [Green Version]
- Daniel, M.C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications Toward Biology, Catalysis, And Nanotechnology. Chem. Rev. 2004, 35, 293–346. [Google Scholar] [CrossRef]
- Vericat, C.; Vela, M.E.; Benitez, G.; Carro, P.; Salvarezza, R.C. Self-assembled monolayers of thiols and dithiols on gold: new challenges for a well-known system. Cheminform 2010, 39, 1805–1834. [Google Scholar]
- Bourg, M.C.; Badia, A.; Lennox, R.B. Gold?Sulfur Bonding in 2D and 3D Self-Assembled Monolayers:? XPS Characterization. J. Phys. Chem. B 2000, 104, 6562–6567. [Google Scholar] [CrossRef]
- Skrzyńska, E.B.; Ftouni, J.; Mamede, A.-S.; Addad, A.; Trentesaux, M.; Girardon, J.-S.; Capron, M.l.; Dumeignil, F. Glycerol oxidation over gold supported catalysts – “Two faces” of sulphur based anchoring agent. J. Mol. Catal. A Chem. 2014, 382, 71–78. [Google Scholar] [CrossRef]









| Linkage Reagent | Original Zeolite (mV) | Silane Deposited Zeolite (mV) | AuNPs Immobilized Zeolite (mV) |
|---|---|---|---|
| APTES | −39.7 ± 2.6 | −36.4 ± 1.6 | −40.2 ± 2.5 |
| MPTES | −39.7 ± 1.8 | −12.2 ± 1.1 | −44.2 ± 2.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, X.; Tatoulian, M.; Guyon, C.; Ognier, S.; Chu, C.; Abou Hassan, A. A Comparison Study of Functional Groups (Amine vs. Thiol) for Immobilizing AuNPs on Zeolite Surface. Nanomaterials 2019, 9, 1034. https://doi.org/10.3390/nano9071034
Rao X, Tatoulian M, Guyon C, Ognier S, Chu C, Abou Hassan A. A Comparison Study of Functional Groups (Amine vs. Thiol) for Immobilizing AuNPs on Zeolite Surface. Nanomaterials. 2019; 9(7):1034. https://doi.org/10.3390/nano9071034
Chicago/Turabian StyleRao, Xi, Michaël Tatoulian, Cédric Guyon, Stephanie Ognier, Chenglin Chu, and Ali Abou Hassan. 2019. "A Comparison Study of Functional Groups (Amine vs. Thiol) for Immobilizing AuNPs on Zeolite Surface" Nanomaterials 9, no. 7: 1034. https://doi.org/10.3390/nano9071034
APA StyleRao, X., Tatoulian, M., Guyon, C., Ognier, S., Chu, C., & Abou Hassan, A. (2019). A Comparison Study of Functional Groups (Amine vs. Thiol) for Immobilizing AuNPs on Zeolite Surface. Nanomaterials, 9(7), 1034. https://doi.org/10.3390/nano9071034