Sustained Release of Basic Fibroblast Growth Factor (bFGF) Encapsulated Polycaprolactone (PCL) Microspheres Promote Angiogenesis In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication and Characterization of bFGF Loaded PCL Microspheres
2.3. In Vitro bFGF Release Studies
2.4. In Vitro Human Endothelial Cell Proliferation Studies
2.5. In Vitro Wound Healing/Scratch Assay to Assess Endothelial Cell Migration
2.6. Gene Expression Analysis of Growth Factors in HUVECs
2.7. In Vivo Angiogenesis Assay
3. Results
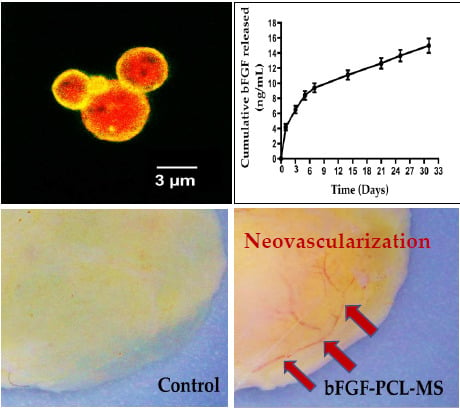
3.1. Fabrication of bFGF-PCL-MS
3.2. In Vitro Release Studies
3.3. In Vitro Cell Proliferation Studies
3.4. In Vitro Wound Healing/Scratch Assay for Assessing Endothelial Cell Migration
3.5. bFGF-PCL-MS Release Media Stimulates Cellular Expression of Angiogenic Genes
3.6. In Vivo Angiogenesis Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics’2017 Update: A Report from the American Heart Association. Circulation 2017. [Google Scholar] [CrossRef] [PubMed]
- van de Werf, F.; Bax, J.; Betriu, A.; Blomstrom-Lundqvist, C.; Crea, F.; Falk, V.; Filippatos, G.; Fox, K.; Huber, K.; Kastrati, A.; et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation. Eur. Heart J. 2008, 29, 2909–2945. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; Reiter, R.J. Cardioprotection and pharmacological therapies in acute myocardial infarction: Challenges in the current era. World J. Cardiol. 2014, 6, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Mulpuru, S.K.; Madhavan, M.; McLeod, C.J.; Cha, Y.M.; Friedman, P.A. Cardiac Pacemakers: Function, Troubleshooting, and Management: Part 1 of a 2-Part Series. J. Am. Coll. Cardiol. 2017, 69, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.K.; Shearier, E.R.; Zhao, S.; Guillory, R.J.; Zhao, F.; Goldman, J.; Drelich, J.W. Biodegradable Metals for Cardiovascular Stents: From Clinical Concerns to Recent Zn-Alloys. Adv. Healthc. Mater. 2016, 5, 1121–1140. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.A.; Wilkoff, B.L. Cardiac Implantable Electronic Device Therapy in Heart Failure. Circ. Res. 2019, 124, 1584–1597. [Google Scholar] [CrossRef]
- Deb, S.; Wijeysundera, H.C.; Ko, D.T.; Tsubota, H.; Hill, S.; Fremes, S.E. Coronary artery bypass graft surgery vs percutaneous interventions in coronary revascularization: A systematic review. JAMA 2013, 310, 2086–2095. [Google Scholar] [CrossRef]
- Kim, I.C.; Youn, J.C.; Kobashigawa, J.A. The past, present and future of heart transplantation. Korean Circ. J. 2018, 48, 565–589. [Google Scholar] [CrossRef]
- Qasim, M.; Arunkumar, P.; Powell, H.M.; Khan, M. Current research trends and challenges in tissue engineering for mending broken hearts. Life Sci. 2019, 229, 233–250. [Google Scholar] [CrossRef]
- Saludas, L.; Pascual-Gil, S.; Roli, F.; Garbayo, E.; Blanco-Prieto, M.J. Heart tissue repair and cardioprotection using drug delivery systems. Maturitas 2018, 110, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rebouças, J.S.; Santos-Magalhães, N.S.; Formiga, F.R. Cardiac Regeneration using Growth Factors: Advances and Challenges. Arq. Bras. Cardiol. 2016, 107, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.D.; Luo, C.Y.; Hu, Y.N.; Yeh, M.L.; Hsueh, Y.C.; Chang, M.Y.; Tsai, D.C.; Wang, J.N.; Tang, M.J.; Wei, E.I.H.; et al. Instructive nanofiber scaffolds with VEGF create a microenvironment for arteriogenesis and cardiac repair. Sci. Transl. Med. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Scheinowitz, M.; Abramov, D.; Eldar, M. The role of insulin-like and basic fibroblast growth factors on ischemic and infarcted myocardium: A mini review. Int. J. Cardiol. 1997, 59, 1–5. [Google Scholar] [CrossRef]
- Hwang, H.; Kloner, R.A. The combined administration of multiple soluble factors in the repair of chronically infarcted rat myocardium. J. Cardiovasc. Pharmcol. 2011, 57, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Tomanek, R.J.; Lotun, K.; Clark, E.B.; Suvarna, P.R.; Hu, N. VEGF and bFGF stimulate myocardial vascularization in embryonic chick. Am. J. Physiol. Circ. Physiol. 2017, 274, H1620–H1626. [Google Scholar] [CrossRef]
- Cross, M.J.; Claesson-Welsh, L. FGF and VEGF function in angiogenesis: Signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. Sci. 2001, 22, 201–207. [Google Scholar] [CrossRef]
- Liu, Z.; Kobayashi, K.; van Dinther, M.; van Heiningen, S.H.; Valdimarsdottir, G.; van Laar, T.; Scharpfenecker, M.; Lowik, C.W.G.M.; Goumans, M.-J.; Dijke, P.t. VEGF and inhibitors of TGFβ type-I receptor kinase synergistically promote blood-vessel formation by inducing α 5-integrin expression. J. Cell Sci. 2009, 122, 3294–3302. [Google Scholar] [CrossRef]
- Yun, Y.R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.H.; Shin, U.S.; Kim, H.W. Fibroblast growth factors: Biology, function, and application for tissue regeneration. J. Tissue Eng. 2010, 1, 218142. [Google Scholar] [CrossRef]
- Yafai, Y.; Iandiev, I.; Lange, J.; Yang, X.M.; Wiedemann, P.A.; Bringmann, W. Eichler, Basic Fibroblast Growth Factor Contributes to a Shift in the Angioregulatory Activity of Retinal Glial (Müller) Cells. PLoS ONE 2013, 8, e68773. [Google Scholar] [CrossRef]
- Kardami, E.; Detillieux, K.; Ma, X.; Jiang, Z.; Santiago, J.J.; Jimenez, S.K.; Cattini, P.A. Fibroblast growth factor-2 and cardioprotection. Heart Fail. Rev. 2007, 12, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Kardami, E.; Jiang, Z.S.; Jimenez, S.K.; Hirst, C.J.; Sheikh, F.; Zahradka, P.; Cattini, P.A. Fibroblast growth factor 2 isoforms and cardiac hypertrophy. Cardiovasc. Res. 2004, 63, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M. FGFs, heparan sulfate and FGFRs: Complex interactions essential for development. Bioessays 2000, 22, 108–112. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, L.; Huan, Y.; Zhao, H.; Deng, J. Application of bFGF and BDNF to Improve Angiogenesis and Cardiac Function. J. Surg. Res. 2006, 136, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Kawasuji, M.; Nagamine, H.; Ikeda, M. Administration of Basic Fibroblast Growth Factor. Ann. Thorac. Surg. 2000, 69, 1155–1161. [Google Scholar] [CrossRef]
- de Marchis, F.; Ribatti, D.; Giampietri, C.; Lentini, A.; Faraone, D.; Scoccianti, M.; Capogrossi, M.C.; Facchiano, A. Platelet-derived growth factor inhibits basic fibroblast growth factor angiogenic properties in vitro and in vivo through its α receptor. Blood 2002, 99, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Li, J.; Li, Y.; Gao, Y.; Zuo, Y.; Hsu, Y.; Hu, J. Angiogenesis and osteogenesis enhanced by bFGF ex vivo gene therapy for bone tissue engineering in reconstruction of calvarial defects. J. Biomed. Mater. Res. Part A 2011, 96, 543–551. [Google Scholar] [CrossRef]
- Shipley, G.D.; Keeble, W.W.; Hendrickson, J.E.; Coffey, R.J.; Pittelkow, M.R. Growth of normal human keratinocytes and fibroblasts in serum-free medium is stimulated by acidic and basic fibroblast growth factor. J. Cell. Physiol. 1989, 138, 511–518. [Google Scholar] [CrossRef]
- Ahn, A.; Frishman, W.H.; Gutwein, A.; Passeri, J.; Nelson, M. Therapeutic Angiogenesis. Cardiol. Rev. 2008, 16, 163–171. [Google Scholar] [CrossRef]
- Senger, D.R.; van de Water, L.; Brown, L.F.; Nagy, J.A.; Yeo, K.T.; Yeo, T.K.; Berse, B.; Jackman, R.W.; Dvorak, A.M.; Dvorak, H.F. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev. 1993, 12, 303–324. [Google Scholar] [CrossRef]
- Cavallaro, U.; Tenan, M.; Castelli, V.; Perilli, A.; Maggiano, N.; van Meir, E.G.; Montesano, R.; Soria, M.R.; Pepper, M.S. Response of bovine endothelial cells to FGF-2 and VEGF is dependent on their site of origin: Relevance to the regulation of angiogenesis. J. Cell. Biochem. 2001, 82, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.M.; Zandstra, P.W. Culture development for human embryonic stem cell propagation: Molecular aspects and challenges. Curr. Opin. Biotechnol. 2005, 16, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Grossman, W.; Friedman, M.; Edelman, E.R.; Prasad, P.V.; Keighley, C.S.; Manning, W.J.; Sellke, F.W.; Simons, M. Basic fibroblast growth factor improves myocardial function in chronically ischemic porcine hearts. J. Clin. Investig. 1994, 94, 623–630. [Google Scholar] [CrossRef] [PubMed]
- House, S.L.; Wang, J.; Castro, A.M.; Weinheimer, C.; Kovacs, A.; Ornitz, D.M. Fibroblast growth factor 2 is an essential cardioprotective factor in a closed-chest model of cardiac ischemia-reperfusion injury. Physiol. Rep. 2015, 3, e12278. [Google Scholar] [CrossRef]
- Aviles, R.J.; Annex, B.H.; Lederman, R.J. Testing clinical therapeutic angiogenesis using basic fibroblast growth factor (FGF-2). Br. J. Pharmacol. 2003, 140, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Bonow, R.O.; Chronos, N.A.; Cohen, D.J.; Giordano, F.J.; Hammond, H.K.; Laham, R.J.; Li, W.; Pike, M.; Sellke, F.W.; et al. Clinical Trials in Coronary Angiogenesis: Issues, Problems, Consensus. Circulation 2012, 102. [Google Scholar] [CrossRef] [PubMed]
- Bentham Science Publisher (B.S.P.). Therapeutic Angiogenesis for Coronary Artery Disease: Clinical Trials of Proteins, Plasmids, Adenovirus and Stem Cells. Stem Cell Regen. Med. 2010, 1, 65–74. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Schmitt, A.; Rödel, P.; Anamur, C.; Seeliger, C.; Imhoff, A.B.; Herbst, E.; Vogt, S.; van Griensven, M.; Winter, G.; Engert, J. Calcium alginate gels as stem cell matrix-making paracrine stem cell activity available for enhanced healing after surgery. PLoS ONE 2015, 10, e0118937. [Google Scholar] [CrossRef]
- Suarez, S.; Grover, G.N.; Braden, R.L.; Christman, K.L.; Almutairi, A. Tunable protein release from acetalated dextran microparticles: A platform for delivery of protein therapeutics to the heart post-MI. Biomacromolecules 2013, 14, 3927–3935. [Google Scholar] [CrossRef]
- Tanihara, M.; Suzuki, Y.; Yamamoto, E.; Noguchi, A.; Mizushima, Y. Sustained release of basic fibroblast growth factor and angiogenesis in a novel covalently crosslinked gel of heparin and alginate. J. Biomed. Mater. Res. 2001, 56, 216–221. [Google Scholar] [CrossRef]
- Shen, H.; Hu, X.; Yang, F.; Bei, J.; Wang, S. Cell affinity for bFGF immobilized heparin-containing poly(lactide-co-glycolide) scaffolds. Biomaterials 2011, 32, 3404–3412. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.K.; Song, Y.L.; Zhou, W.; Yu, M.; Liang, L.H.; Sun, D.C.; Li, D.H.; Deng, Z.X.; Zhu, W.Z. Effects of local delivery of bFGF from PLGA microspheres on osseointegration around implants in diabetic rats. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Gao, J.; Chen, C.-W.; Huard, J.; Wang, Y. Injectable fibroblast growth factor-2 coacervate for persistent angiogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 13444–13449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.W.; Zhang, Y.F.; Sun, Z.Y.; Song, G.T.; Chen, Z. Dental pulp tissue engineering with bFGF-incorporated silk fibroin scaffolds. J. Biomater. Appl. 2015, 30, 221–229. [Google Scholar] [CrossRef]
- Chen, W.C.W.; Lee, B.G.; Park, D.W.; Kim, K.; Chu, H.; Kim, K.; Huard, J.; Wang, Y. Controlled dual delivery of fibroblast growth factor-2 and Interleukin-10 by heparin-based coacervate synergistically enhances ischemic heart repair. Biomaterials 2015, 72, 138–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosaka, A.; Koyama, H.; Kushibiki, T.; Tabata, Y.; Nishiyama, N.; Miyata, T.; Shigematsu, H.; Takato, T.; Nagawa, H. Gelatin hydrogel microspheres enable pinpoint delivery of basic fibroblast growth factor for the development of functional collateral vessels. Circulation 2004, 110, 3322–3328. [Google Scholar] [CrossRef]
- Shen, B.; Pei, F.X.; Duan, H.; Chen, J.; Mu, J.X. Preparation and in vitro activity of controlled release microspheres incorporating bFGF. Chin. J. Traumatol. 2008, 11, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wu, G.; Bai, S.; Feng, Z.; Dong, Y.; Zhou, J.; Qin, H.; Zhao, Y. MAPs/bFGF-PLGA microsphere composite-coated titanium surfaces promote increased adhesion and proliferation of fibroblasts. Biomed. Mater. 2014, 9. [Google Scholar] [CrossRef]
- Liu, H.; Fan, H.; Cui, Y.; Chen, Y.; Yao, K.; Goh, J.C.H. Effects of the controlled-released basic fibroblast growth factor from chitosan-Gelatin microspheres on human fibroblasts cultured on a chitosan-Gelatin scaffold. Biomacromolecules 2007, 8, 1446–1455. [Google Scholar] [CrossRef]
- Lv, B.; Wang, Y.; Chen, W. Preparation, Characterization, and Bioactivity of Chitosan Microspheres Containing Basic Fibroblast Growth Factor. J. Nanomater. 2014, 2014, 163. [Google Scholar] [CrossRef]
- Ali, Z.; Islam, A.; Sherrell, P.; Le-Moine, M.; Lolas, G.; Syrigos, K.; Rafat, M.; Jensen, L.D. Adjustable delivery of pro-angiogenic FGF-2 by alginate:collagen microspheres. Biol. Open 2018, 7, bio027060. [Google Scholar] [CrossRef]
- Sakakibara, Y.; Tambara, K.; Sakaguchi, G.; Lu, F.; Yamamoto, M.; Nishimura, K.; Tabata, Y.; Komeda, M. Toward surgical angiogenesis using slow-released basic fibroblast growth factor. Eur. J. Cardio-Thorac. Surg. 2003, 24, 105–112. [Google Scholar] [CrossRef]
- Yamamoto, T.; Suto, N.; Okubo, T.; Mikuniya, A.; Hanada, H.; Yagihashi, S.; Fujita, M.; Okumura, K. Intramyocardial Delivery of Basic Fibroblast Growth Factor-Impregnated Gelatin Hydrogel Microspheres Enhances Collateral Circulation to Infarcted Canine Myocardium. Jpn. Circ. J. 2001, 65, 439–444. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Wong, D.Y.; Hollister, S.J.; Krebsbach, P.H.; Nosrat, C. Poly(ɛ-Caprolactone) and Poly (L-Lactic-Co-Glycolic Acid) Degradable Polymer Sponges Attenuate Astrocyte Response and Lesion Growth in Acute Traumatic Brain Injury. Tissue Eng. 2007, 13, 2515–2523. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. Progress in Polymer Science The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. International Journal of Polymeric Materials and Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Sukanya, V.S.; Mohanan, P.V. Degradation of Poly(ε-caprolactone) and bio-interactions with mouse bone marrow mesenchymal stem cells. Colloids Surf. B Biointerfaces 2018, 163, 107–118. [Google Scholar] [CrossRef]
- Shamloo, A.; Sarmadi, M.; Aghababaie, Z.; Vossoughi, M. Accelerated full-thickness wound healing via sustained bFGF delivery based on a PVA/chitosan/gelatin hydrogel incorporating PCL microspheres. Int. J. Pharm. 2018, 537, 278–289. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Michael, W.P. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29. [Google Scholar] [CrossRef]
- Khan, M.; Xu, Y.; Hua, S.; Johnson, J.; Belevych, A.; Janssen, P.M.L.; Gyorke, S.; Guan, J.; Angelos, M.G. Evaluation of changes in morphology and function of human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CMs) cultured on an aligned-nanofiber cardiac patch. PLoS ONE 2015, 10, e0126338. [Google Scholar] [CrossRef]
- Deveza, L.; Choi, J.; Yang, F. Therapeutic angiogenesis for treating cardiovascular diseases. Theranostics 2012, 2, 801–814. [Google Scholar] [CrossRef]
- Buschmann, I.; Schaper, W. The pathophysiology of the collateral circulation (arteriogenesis). J. Pathol. 2000, 190, 338–342. [Google Scholar] [CrossRef]
- Dudley, A.C.; Claesson-Welsh, L. Mechanisms of angiogenesis and lymphangiogenesis. Tumor Angiogenes 2010, 1, 17–34. [Google Scholar]
- van Royen, N.; Piek, J.J.; Buschmann, I.; Hoefer, I.; Voskuil, M.; Schaper, W. Stimulation of arteriogenesis; a new concept for the treatment of arterial occlusive disease. Cardiovasc. Res. 2001, 49, 543–553. [Google Scholar] [CrossRef]
- Wahlberg, E. Angiogenesis and arteriogenesis in limb ischemia. J. Vasc. Surg. 2003, 38, 198–203. [Google Scholar] [CrossRef] [Green Version]
- Helisch, A.; Schaper, W. Arteriogenesis: The development and growth of collateral arteries. Microcirculation 2003, 10, 83–97. [Google Scholar] [CrossRef]
- Deindl, E.; Hoefer, I.E.; Fernandez, B.; Barancik, M.; Heil, M.; Strniskova, M.; Schaper, W. Involvement of the fibroblast growth factor system in adaptive and chemokine-induced arteriogenesis. Circ. Res. 2003, 92, 561–568. [Google Scholar] [CrossRef]
- Doukas, J.; Blease, K.; Craig, D.; Ma, C.; Chandler, L.A.; Sosnowski, B.A.; Pierce, G.F. Delivery of FGF genes to wound repair cells enhances arteriogenesis and myogenesis in skeletal muscle. Mol. Ther. 2002, 5, 517–527. [Google Scholar] [CrossRef]
- Chung, H.J.; Kim, H.K.; Yoon, J.J.; Park, T.G. Heparin immobilized porous PLGA microspheres for angiogenic growth factor delivery. Pharm. Res. 2006, 23, 1835–1841. [Google Scholar] [CrossRef]
- Wolfe, A.; O’Clair, B.; Groppi, V.E.; McEwen, D.P. Pharmacologic characterization of a kinetic in vitro human co-culture angiogenesis model using clinically relevant compounds. J. Biomol. Screen. 2013, 18, 1234–1245. [Google Scholar] [CrossRef]
- Shi, B.; Andrukhov, O.; Berner, S.; Schedle, A.; Rausch-Fan, X. The angiogenic behaviors of human umbilical vein endothelial cells (HUVEC) in co-culture with osteoblast-like cells (MG-63) on different titanium surfaces. Dent. Mater. 2014, 30, 839–847. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arunkumar, P.; Dougherty, J.A.; Weist, J.; Kumar, N.; Angelos, M.G.; Powell, H.M.; Khan, M. Sustained Release of Basic Fibroblast Growth Factor (bFGF) Encapsulated Polycaprolactone (PCL) Microspheres Promote Angiogenesis In Vivo. Nanomaterials 2019, 9, 1037. https://doi.org/10.3390/nano9071037
Arunkumar P, Dougherty JA, Weist J, Kumar N, Angelos MG, Powell HM, Khan M. Sustained Release of Basic Fibroblast Growth Factor (bFGF) Encapsulated Polycaprolactone (PCL) Microspheres Promote Angiogenesis In Vivo. Nanomaterials. 2019; 9(7):1037. https://doi.org/10.3390/nano9071037
Chicago/Turabian StyleArunkumar, Pala, Julie A. Dougherty, Jessica Weist, Naresh Kumar, Mark G. Angelos, Heather M. Powell, and Mahmood Khan. 2019. "Sustained Release of Basic Fibroblast Growth Factor (bFGF) Encapsulated Polycaprolactone (PCL) Microspheres Promote Angiogenesis In Vivo" Nanomaterials 9, no. 7: 1037. https://doi.org/10.3390/nano9071037
APA StyleArunkumar, P., Dougherty, J. A., Weist, J., Kumar, N., Angelos, M. G., Powell, H. M., & Khan, M. (2019). Sustained Release of Basic Fibroblast Growth Factor (bFGF) Encapsulated Polycaprolactone (PCL) Microspheres Promote Angiogenesis In Vivo. Nanomaterials, 9(7), 1037. https://doi.org/10.3390/nano9071037