Synthesis of Hierarchical Porous Carbon in Molten Salt and Its Application for Dye Adsorption
Abstract
:1. Introduction
2. Materials and Methods
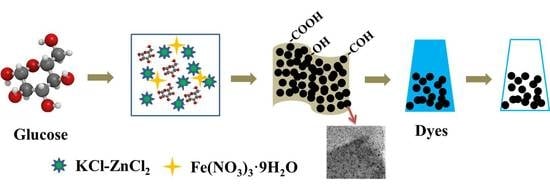
2.1. Raw Materials and Sample Preparation
2.2. Adsorption Test
2.3. Characterization
3. Results and Discussion
3.1. Microstructural Characterization of As-Prepared Porous Carbon
3.2. Adsorption Performance of As-Prepared Porous Carbon for Methylene Blue and Methyl Orange
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siyasukh, A.; Chimupala, Y.; Tonanon, N. Preparation of magnetic hierarchical porous carbon spheres with graphitic features for high methyl orange adsorption capacity. Carbon 2018, 134, 207–221. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S. Dye and its removal from aqueous solution by adsorption. Adv. Colloid Interfac. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Başar, C.A. Applicability of the various adsorption models of three dyes adsorption onto activated carbon prepared waste apricot. J. Hazard Mater. 2006, 135, 232–241. [Google Scholar] [CrossRef]
- Öner, Ş.G.; Kabay, N.; Güler, E. A comparative study for the removal of boron and silica from geothermal water by cross-flow flat sheet reverse osmosis method. Desalination 2011, 283, 10–15. [Google Scholar] [CrossRef]
- Murugananthan, M.; Raju, G.B.; Prabhakar, S. Separation of pollutants from tannery effluents by electro flotation. Sep. Purif. Technol. 2004, 40, 69–75. [Google Scholar] [CrossRef]
- Dutta, K.; Mukhopadhyay, S.; Bhattacharjee, S. Chemical oxidation of methylene blue using a Fenton-like reaction. J. Hazard Mater. 2001, 84, 57–71. [Google Scholar] [CrossRef]
- Vilensky, M.Y.; Berkowitz, B.; Warshawsky, A. In Situ remediation of groundwater contaminated by heavy- and transition-metal ions by selective ion-exchange methods. Environ. Sci. Technol. 2002, 36, 1851–1855. [Google Scholar] [CrossRef]
- Huq, A.; Xu, B.; Chowdhury, M.A. A simple filtration method to remove plankton-associated Vibrio cholerae in raw water supplies in developing countries. Appl. Environ. Microb. 1996, 62, 2508–2512. [Google Scholar]
- SariciÖzdemir, Ç.; Önal, Y. Study to observe the applicability of the adsorption isotherms used for the adsorption of medicine organics onto activated carbon. Part. Sci. Technol. 2018, 36, 254–261. [Google Scholar] [CrossRef]
- Önal, Y.; Akmil-Başar, C.; Sarıcı-Özdemir, Ç. Elucidation of the naproxen sodium adsorption onto activated carbon prepared from waste apricot: Kinetic, equilibrium and thermodynamic characterization. J. Hazard Mater. 2007, 148, 727–734. [Google Scholar] [CrossRef]
- Önal, Y.; Akmil-Başar, C.; Eren, D. Adsorption kinetics of malachite green onto activated carbon prepared from Tunçbilek lignite. J. Hazard Mater. 2006, 128, 150–157. [Google Scholar] [CrossRef]
- Gu, L.; Zhu, N.; Guo, H. Adsorption and Fenton-like degradation of naphthalene dye intermediate on sewage sludge derived porous carbon. J. Hazard Mater. 2013, 246–247, 145–153. [Google Scholar] [CrossRef]
- Lei, Z.; Xiao, Y.; Dang, L. Graphitized carbon with hierarchical mesoporous structure templated from colloidal silica particles. Microporous Mesoporous Mater. 2008, 109, 109–117. [Google Scholar] [CrossRef]
- Gorka, J.; Zawislak, A.; Choma, J.; Jaroniec, M. KOH activation of mesoporous carbons obtained by soft-templating. Carbon 2008, 46, 1159–1161. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Dai, Q. Preparation of novel porous carbon spheres from corn starch. Colloid Surface A 2009, 346, 213–215. [Google Scholar] [CrossRef]
- Reddy, M.V.; Yu, C.; Jiahuan, F. Molten salt synthesis and energy storage studies on CuCo2O4 and CuO·Co3O4. RSC Adv. 2012, 2, 9619–9625. [Google Scholar] [CrossRef]
- Reddy, M.V.; Beichen, Z.; Nicholette, L.J.E.; Kaimeng, Z.; Chowdari, B.V.R. Molten salt synthesis and its electrochemical characterization of Co3O4 for lithium batteries. Electrochem. Solid State Lett. 2011, 14, 79–82. [Google Scholar] [CrossRef]
- Reddy, M.V.; Cherian, C.T.; Ramanathan, K. Molten synthesis of ZnO·Fe3O4 and Fe2O3 and its electrochemical performance. Electrochimica Acta 2014, 118, 75–80. [Google Scholar] [CrossRef]
- Reddy, M.V.; Teoh, X.W.V.; Nguyen, T.B. Effect of 0.5 M NaNO3: 0.5 M KNO3 and 0.88 M LiNO3: 0.12 M LiCl molten salts. and heat treatment on electrochemical properties of TiO2. J. Electrochem. Soc. 2012, 159, 762–769. [Google Scholar] [CrossRef]
- Nithyadharseni, P.; Reddy, M.V.; Ozoemena, K.I. Low temperature molten salt synthesis of Y2Sn2O7 anode material for lithium ion batteries. Electrochim. Acta 2015, 182, 1060–1069. [Google Scholar] [CrossRef]
- Reddy, M.V.; Tung, B.D.; Yang, L. Molten salt method of preparation and cathodic studies on layered-cathode materials Li(Co0.7Ni0.3)O2 and Li(Ni0.7Co0.3)O2 for Li-ion batteries. J. Power Sources 2013, 225, 374–381. [Google Scholar] [CrossRef]
- Reddy, M.V.; Beichen, Z.; Loh, K.P. Facile synthesis of Co3O4 by molten salt method and its Li-storage performance. CrystEngComm 2013, 15, 3568–3574. [Google Scholar] [CrossRef]
- Tan, K.S.; Reddy, M.V.; Rao, G.V. High-performance LiCoO2 by molten salt (LiNO3: LiCl) synthesis for Li-ion batteries. J. Power Sources 2015, 147, 241–248. [Google Scholar] [CrossRef]
- Reddy, M.V.; Rao, G.V.S.; Chowdari, B.V.R. Synthesis by molten salt and cathodic properties of Li(Ni1/3Co1/3Mn1/3)O2. J. Power Sources 2006, 159, 263–267. [Google Scholar] [CrossRef]
- Reddy, M.V.; Rao, G.V.S.; Chowdari, B.V.R. Synthesis and electrochemical studies of the 4V cathode, Li(Ni2/3Mn1/3)O2. J. Power Sources 2006, 160, 1369–1374. [Google Scholar] [CrossRef]
- Hudry, D.; Rakhmatullin, A.; Bessada, C. Reactivity of NH4H2PO4 toward LaCl3 in LiCl-KCl melt flux step by step formation of monazite-Like LaPO4. Inorg. Chem. 2009, 48, 7141–7150. [Google Scholar] [CrossRef]
- Portehault, D.; Devi, S.; Beaunier, P. A general solution route toward metal boride nanocrystals. Angew. Chem. Int. Ed. 2011, 123, 3320–3323. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, L.; Liang, F. Facile synthesis of hexagonal boron nitride nanoplates via molten-salt-mediated magnesiothermic reduction. Ceram. Int. 2015, 41, 14941–14948. [Google Scholar] [CrossRef]
- Liang, F.; Tian, L.; Zhang, H. Low temperature synthesis of LiSi2N3 nanobelts via molten salt nitridation and their photoluminescence properties. RSC Adv. 2016, 6, 68615–68618. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Z.; Huo, C. Low-temperature rapid synthesis of rod-like ZrB2 powders by molten-salt and microwave Co-assisted carbothermal reduction. J. Am. Ceram. Soc. 2016, 99, 2895–2898. [Google Scholar] [CrossRef]
- Huang, Z.; Duan, H.; Liu, J. Preparation of lanthanum cerate powders via a simple molten salt route. Ceram. Int. 2016, 42, 10482–10486. [Google Scholar] [CrossRef]
- Liu, X.; Antonietti, M. Molten salt activation for synthesis of porous carbon nanostructures and carbon sheets. Carbon 2014, 69, 460–466. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, B.; Zhu, L. Molten salt synthesis of nitrogen-doped carbon with hierarchical pore structures for use as high-performance electrodes in supercapacitors. Carbon 2015, 93, 48–58. [Google Scholar] [CrossRef]
- Petnikota, S.; Maseed, H.; Srikanth, V. Experimental elucidation of a graphenothermal reduction mechanism of Fe2O3: An enhanced anodic behavior of an exfoliated reduced graphene oxide/Fe3O4 composite in Li-ion batteries. J. Phys. Chem. C 2017, 121, 3778–3789. [Google Scholar] [CrossRef]
- Das, B.; Reddy, M.V.; Chowdari, B.V.R. Li-storage of Fe3O4/C composite prepared by one-step carbothermal reduction method. J. Alloy Compd. 2013, 565, 90–96. [Google Scholar] [CrossRef]
- Li, S.; Liang, F.; Wang, J. Preparation of mono-dispersed carbonaceous spheres via hydrothermal process. Adv. Powder Technol. 2017, 28, 2648–2657. [Google Scholar] [CrossRef]
- Tian, L.; Li, J.; Liang, F. Molten salt synthesis of tetragonal carbon nitride hollow tubes and their application for removal of pollutants from wastewater. Appl. Catal. B Environ. 2017, 225, 307–313. [Google Scholar] [CrossRef]
- Gao, H.; Yan, S.; Wang, J. Towards efficient solar hydrogen production by intercalated carbon nitride photocatalyst. Phys. Chem. Chem. Phys. 2013, 15, 18077–18084. [Google Scholar] [CrossRef]
- Sevilla, M.; Ferrero, G.A.; Fuertes, A.B. One-pot synthesis of biomass-based hierarchical porous carbons with a large porosity development. Chem. Mater. 2017, 29, 6900–6907. [Google Scholar] [CrossRef]
- Ma, C.; Chen, X.; Long, D. High-surface-area and high-nitrogen-content carbon microspheres prepared by a pre-oxidation and mild KOH activation for superior supercapacitor. Carbon 2017, 118, 699–708. [Google Scholar] [CrossRef] [Green Version]
- Estevez, L.; Prabhakaran, V.; Garcia, A.L. Hierarchically porous graphitic carbon with simultaneously high surface area and colossal pore volume engineered via ice templating. ACS Nano 2017, 11, 11047–11055. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Meng, F.; Li, J. Carbonized design of hierarchical porous carbon/Fe3O4@Fe derived from loofah sponge to achieve tunable high-performance microwave absorption. ACS Sustain. Chem. Eng. 2018, 11, 11801–11810. [Google Scholar] [CrossRef]
- Li, P.; Bai, G.; Liu, T. Preparation of nano-Fe2O3/C supercapacitor electrode material by solution combustion method. New Chem. Mater. 2015, 9, 48–50. [Google Scholar]
- Islam, M.A.; Ahmed, M.J.; Khanday, W.A. Mesoporous activated carbon prepared from NaOH activation of rattan (Lacosperma secundiflorum) hydrochar for methylene blue removal. Ecotoxicol. Environ. Saf. 2017, 138, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies. J. Hazard Mater. 2008, 154, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Önal, Y.; Akmil-Başar, C.; Sarıcı-Özdemir, Ç. Investigation kinetics mechanisms of adsorption malachite green onto activated carbon. J. Hazard Mater. 2007, 146, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, J.; Zhang, H. Use of rice husk-based porous carbon for adsorption of rhodamine B from aqueous solutions. Dyes Pigment. 2005, 66, 123–128. [Google Scholar] [CrossRef]
- Chen, Z.; Yingjie, L.; Weihao, Z. Synthesis and Zn(II) modification of hierarchical porous carbon materials from petroleum pitch for effective adsorption of organic dyes. Chemosphere 2019, 216, 379–386. [Google Scholar]
- Hao, Y.; Wang, Z.; Wang, Z. Preparation of hierarchically porous carbon from cellulose as highly efficient adsorbent for the removal of organic dyes from aqueous solutions. Ecotoxicol. Environ. Saf. 2019, 168, 298–303. [Google Scholar] [CrossRef]






| Sample | SBET (m2/g) | Vtotal (cm3/g) | Vmeso (cm3/g) | Vmeso/Vtotal (%) |
|---|---|---|---|---|
| 0 Fe | 753 | 0.453 | 0.141 | 31.1 |
| 0.5 Fe | 817 | 0.483 | 0.173 | 35.8 |
| 1.0 Fe | 1078 | 0.636 | 0.375 | 59.0 |
| 1.5 Fe | 903 | 0.586 | 0.357 | 60.9 |
| 2.0 Fe | 767 | 0.484 | 0.183 | 37.8 |
| Sample | Dyes | Langmuir | Freundlich | Pseudo-First-Order | Pseudo-Second-Order | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q0 | B | R2 | KF | N | R2 | Qe | K1 | R2 | Qe | K2 | R2 | ||
| 0 Fe | MB | 188.4 | 0.031 | 0.83 | 1.6 | 1.15 | 0.91 | 124.8 | 0.50 | 0.995 | 142.9 | 0.016 | 0.999 |
| 0.5 Fe | 412 | 0.012 | 0.94 | 1.5 | 1.01 | 0.95 | 240 | 0.59 | 0.992 | 250 | 0.012 | 0.999 | |
| 1.0 Fe | 506.8 | 0.009 | 0.96 | 1.4 | 0.99 | 0.96 | 274.7 | 0.59 | 0.995 | 333.3 | 0.009 | 0.998 | |
| 1.5 Fe | 442.5 | 0.011 | 0.95 | 1.4 | 1.0 | 0.95 | 260.9 | 0.41 | 0.993 | 277.8 | 0.005 | 0.999 | |
| 2.0 Fe | 194 | 0.029 | 0.84 | 1.6 | 1.2 | 0.91 | 127 | 0.50 | 0.996 | 142.9 | 0.012 | 0.998 | |
| Sample | Dyes | Langmuir | Freundlich | Pseudo-First-Order | Pseudo-Second-Order | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q0 | B | R2 | KF | N | R2 | Qe | K1 | R2 | Qe | K2 | R2 | ||
| 0 Fe | MO | 249.5 | 0.020 | 0.95 | 1.7 | 1.16 | 0.91 | 161.5 | 0.58 | 0.998 | 166.7 | 0.016 | 0.999 |
| 0.5 Fe | 559 | 0.008 | 0.98 | 1.5 | 1.02 | 0.95 | 311.9 | 0.50 | 0.995 | 333.3 | 0.005 | 0.999 | |
| 1.0 Fe | 683.8 | 0.007 | 0.96 | 1.1 | 0.99 | 0.94 | 370.7 | 0.60 | 0.995 | 379 | 0.007 | 0.998 | |
| 1.5 Fe | 521 | 0.007 | 0.96 | 1.4 | 1.0 | 0.95 | 334.1 | 0.56 | 0.993 | 344.8 | 0.006 | 0.999 | |
| 2.0 Fe | 254 | 0.020 | 0.98 | 1.8 | 1.2 | 0.90 | 166.8 | 0.51 | 0.996 | 172.4 | 0.013 | 0.999 | |
| Adsorbent | Dyes | T (°C) | BET (m2/g) | Qm (mg/g) | Reference |
|---|---|---|---|---|---|
| Mesoporous activated carbon | MB | 30 | 1135 | 359 | [44] |
| Activated carbon | MB | 30~50 | 1940 | 434.78 | [45] |
| Activated carbon | MB | 30~50 | 1060 | 102.04 | [3] |
| Activated carbon | MG | 25~50 | 1000 | 149 | [46] |
| Porous carbon | RB | - | 2721 | 479 | [47] |
| Hierarchical porous carbon materials | MB/DB | 25 | 1913 | 1585.7/438.6 | [48] |
| Hierarchical porous carbon materials | MO | 25 | 1338.9 | 598.8 | [49] |
| Hierarchical porous carbon materials | MB/MO | 25 | 1078 | 506.8/683.8 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zhang, H.; Hu, S.; Liu, J.; Zhu, Q.; Zhang, S. Synthesis of Hierarchical Porous Carbon in Molten Salt and Its Application for Dye Adsorption. Nanomaterials 2019, 9, 1098. https://doi.org/10.3390/nano9081098
Li S, Zhang H, Hu S, Liu J, Zhu Q, Zhang S. Synthesis of Hierarchical Porous Carbon in Molten Salt and Its Application for Dye Adsorption. Nanomaterials. 2019; 9(8):1098. https://doi.org/10.3390/nano9081098
Chicago/Turabian StyleLi, Saisai, Haijun Zhang, Shiya Hu, Jie Liu, Qing Zhu, and Shaowei Zhang. 2019. "Synthesis of Hierarchical Porous Carbon in Molten Salt and Its Application for Dye Adsorption" Nanomaterials 9, no. 8: 1098. https://doi.org/10.3390/nano9081098
APA StyleLi, S., Zhang, H., Hu, S., Liu, J., Zhu, Q., & Zhang, S. (2019). Synthesis of Hierarchical Porous Carbon in Molten Salt and Its Application for Dye Adsorption. Nanomaterials, 9(8), 1098. https://doi.org/10.3390/nano9081098