Synthesis of Three-Dimensional Graphene-Based Hybrid Materials for Water Purification: A Review
Abstract
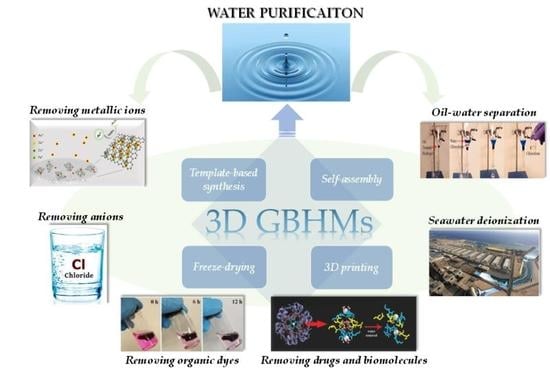
:1. Introduction
2. Fabrication Methods of 3D GBHMs
2.1. Template-Based Synthesis
2.2. Self-Assembly
2.3. Freeze-Drying
2.4. 3D Printing
3. Techniques for Water Purification
3.1. Filtration
3.2. Adsorption and Removal
3.3. Photocatalytic Degradation
3.4. Electrocatalytic Degradation
3.5. Biodegradation
3.6. Capacitive Deionization
4. 3D GBHMs for Water Purification Applications
4.1. Removing Metallic Ions
4.2. Removing Anions
4.3. Removing Organic Dyes
4.4. Removing Drugs and Biomolecules
4.5. Oil-Water Separation
4.6. Seawater Deionization
5. Conclusions and Outlooks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Ko, S.H.; Kang, K.H.; Han, J. Direct seawater desalination by ion concentration polarization. Nat. Nanotechnol. 2010, 5, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Deng, B.L. Polymer-matrix nanocomposite membranes for water treatment. J. Membr. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Lee, J.W.; Jung, J.; Cho, Y.H.; Yadav, S.K.; Baek, K.Y.; Park, H.B.; Hong, S.M.; Koo, C.M. Fouling-Tolerant Nanofibrous Polymer Membranes for Water Treatment. ACS Appl. Mater. Interfaces 2014, 6, 14600–14607. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Xu, J.J.; Ozaydin-Ince, G.; Wong, S.Y.; Gleason, K.K. Surface-Tethered Zwitterionic Ultrathin Antifouling Coatings on Reverse Osmosis Membranes by Initiated Chemical Vapor Deposition. Chem. Mater. 2011, 23, 1263–1272. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Wu, A.G.; Colombi Ciacchi, L.; Wei, G. Recent advances in nanoporous membranes for water purification. Nanomaterials 2018, 8, 65. [Google Scholar] [CrossRef]
- Ali, I.; Gupta, V. Advances in water treatment by adsorption technology. Nat. Protoc. 2006, 1, 2661. [Google Scholar] [CrossRef]
- Daigle, T. Ultra Deep Water Discharge of Produced Water and/or Solids at the Seabed; Report 09121-3100-01; RPSEA: Houston, TX, USA, 2012. [Google Scholar]
- Çeçen, F.; Aktas, Ö. Activated Carbon for Water and Wastewater Treatment: INTEGRATION of Adsorption and Biological Treatment; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Xi, Y.; Mallavarapu, M.; Naidu, R. Preparation, characterization of surfactants modified clay minerals and nitrate adsorption. Appl. Clay Sci. 2010, 48, 92–96. [Google Scholar] [CrossRef] [Green Version]
- Qi, P.; Pichler, T. Competitive adsorption of As (III), As (V), Sb (III) and Sb (V) onto ferrihydrite in multi-component systems: Implications for mobility and distribution. J. Hazard. Mater. 2017, 330, 142–148. [Google Scholar] [CrossRef]
- Li, M.; Zhu, X.; Zhu, F.; Ren, G.; Cao, G.; Song, L. Application of modified zeolite for ammonium removal from drinking water. Desalination 2011, 271, 295–300. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211, 317–331. [Google Scholar] [CrossRef]
- Qi, P.; Luo, R.; Pichler, T.; Zeng, J.; Wang, Y.; Fan, Y.; Sui, K. Development of a magnetic core-shell Fe3O4@ TA@ UiO-66 microsphere for removal of arsenic (III) and antimony (III) from aqueous solution. J. Hazard. Mater. 2019. [Google Scholar] [CrossRef]
- Mallampati, R.; Valiyaveettil, S. Apple Peels—A Versatile Biomass for Water Purification? ACS Appl. Mater. Interfaces 2013, 5, 4443–4449. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Mittal, H.; Maity, A.; Ray, S.S. Gum karaya based hydrogel nanocomposites for the effective removal of cationic dyes from aqueous solutions. Appl. Surf. Sci. 2016, 364, 917–930. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef]
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef]
- Sarkar, B.; Mandal, S.; Tsang, Y.F.; Kumar, P.; Kim, K.-H.; Ok, Y.S. Designer carbon nanotubes for contaminant removal in water and wastewater: A critical review. Sci. Total Environ. 2018, 612, 561–581. [Google Scholar] [CrossRef]
- Jiuhui, Q. Research progress of novel adsorption processes in water purification: A review. J. Environ. Sci. 2008, 20, 1–13. [Google Scholar]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Wang, L.; Wu, A.G.; Wei, G. Graphene-based aptasensors: From molecule-interface interactions to sensor design and biomedical diagnostics. Analyst 2018, 143, 1526–1543. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.J.; Wu, A.G.; Wei, G. Designed graphene-peptide nanocomposites for biosensor applications: A review. Anal. Chim. Acta 2017, 985, 24–40. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Ciacchi, L.C.; Wei, G. Recent Advances in the Synthesis of Graphene-Based Nanomaterials for Controlled Drug Delivery. Appl. Sci. 2017, 7, 1175. [Google Scholar] [CrossRef]
- Szabó, T.; Péter, Z.; Illés, E.; Janovák, L.; Talyzin, A. Stability and dye inclusion of graphene oxide/polyelectrolyte layer-by-layer self-assembled films in saline, acidic and basic aqueous solutions. Carbon 2017, 111, 350–357. [Google Scholar] [CrossRef]
- Szabó, T.; Szeri, A.; Dékány, I. Composite graphitic nanolayers prepared by self-assembly between finely dispersed graphite oxide and a cationic polymer. Carbon 2005, 43, 87–94. [Google Scholar] [CrossRef]
- Szabó, T.; Bakandritsos, A.; Tzitzios, V.; Devlin, E.; Petridis, D.; Dékány, I. Magnetically modified single and turbostratic stacked graphenes from tris (2, 2′-bipyridyl) iron (II) ion-exchanged graphite oxide. J. Phys. Chem. B 2008, 112, 14461–14469. [Google Scholar] [CrossRef]
- Joshi, R.K.; Carbone, P.; Wang, F.C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.A.; Geim, A.K.; Nair, R.R. Precise and Ultrafast Molecular Sieving Through Graphene Oxide Membranes. Science 2014, 343, 752–754. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.J.; Lv, W.; Yang, Q.H. Self-assembly of graphene oxide at interfaces. Adv. Mater. 2014, 26, 5586–5612. [Google Scholar] [CrossRef]
- Yao, H.B.; Tan, Z.H.; Fang, H.Y.; Yu, S.H. Artificial nacre-like bionanocomposite films from the self-assembly of chitosan–montmorillonite hybrid building blocks. Angew. Chem. Int. Ed. 2010, 49, 10127–10131. [Google Scholar] [CrossRef]
- Han, Y.; Xu, Z.; Gao, C. Ultrathin Graphene Nanofiltration Membrane for Water Purification. Adv. Funct. Mater. 2013, 23, 3693–3700. [Google Scholar] [CrossRef]
- Sun, P.Z.; Zhu, M.; Wang, K.L.; Zhong, M.L.; Wei, J.Q.; Wu, D.H.; Xu, Z.P.; Zhu, H.W. Selective Ion Penetration of Graphene Oxide Membranes. ACS Nano 2013, 7, 428–437. [Google Scholar] [CrossRef]
- Li, C.; Shi, G.Q. Three-dimensional graphene architectures. Nanoscale 2012, 4, 5549–5563. [Google Scholar] [CrossRef]
- Dasgupta, A.; Rajukumar, L.P.; Rotella, C.; Lei, Y.; Terrones, M. Covalent three-dimensional networks of graphene and carbon nanotubes: Synthesis and environmental applications. Nano Today 2017, 12, 116–135. [Google Scholar] [CrossRef]
- Qiu, B.C.; Xing, M.Y.; Zhang, J.L. Recent advances in three-dimensional graphene based materials for catalysis applications. Chem. Soc. Rev. 2018, 47, 2165–2216. [Google Scholar] [CrossRef]
- Wu, Y.P.; Zhu, J.H.; Huang, L. A review of three-dimensional graphene-based materials: Synthesis and applications to energy conversion/storage and environment. Carbon 2019, 143, 610–640. [Google Scholar] [CrossRef]
- Shen, Y.; Fang, Q.L.; Chen, B.L. Environmental Applications of Three-Dimensional Graphene-Based Macrostructures: Adsorption, Transformation, and Detection. Environ. Sci. Technol. 2015, 49, 67–84. [Google Scholar] [CrossRef]
- Luo, J.S.; Liu, J.L.; Zeng, Z.Y.; Ng, C.F.; Ma, L.J.; Zhang, H.; Lin, J.Y.; Shen, Z.X.; Fan, H.J. Three-Dimensional Graphene Foam Supported Fe3O4 Lithium Battery Anodes with Long Cycle Life and High Rate Capability. Nano Lett. 2013, 13, 6136–6143. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Guo, S.J.; Zhai, Y.M.; Dong, S.J. Layer-by-Layer Self-Assembly for Constructing a Graphene/Platinum Nanoparticle Three-Dimensional Hybrid Nanostructure Using Ionic Liquid as a Linker. Langmuir 2010, 26, 7614–7618. [Google Scholar] [CrossRef]
- Ye, S.B.; Feng, J.C.; Wu, P.Y. Highly elastic graphene oxide-epoxy composite aerogels via simple freeze-drying and subsequent routine curing. J. Mater. Chem. A 2013, 1, 3495–3502. [Google Scholar] [CrossRef]
- Wei, X.J.; Li, D.; Jiang, W.; Gu, Z.M.; Wang, X.J.; Zhang, Z.X.; Sun, Z.Z. 3D Printable Graphene Composite. Sci. Rep. 2015, 5, 11181. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Li, T.; Deng, S.; Cen, K.; Xu, L.; Tang, Y. A General Strategy for the Synthesis of PtM (M = Fe, Co, Ni) Decorated Three-Dimensional Hollow Graphene Nanospheres for Efficient Methanol Electrooxidation. Chem. A Eur. J. 2018, 24, 1246–1252. [Google Scholar] [CrossRef]
- Chen, Z.P.; Ren, W.C.; Gao, L.B.; Liu, B.L.; Pei, S.F.; Cheng, H.M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef]
- Cao, X.H.; Shi, Y.M.; Shi, W.H.; Rui, X.H.; Yan, Q.Y.; Kong, J.; Zhang, H. Preparation of MoS2-Coated Three-Dimensional Graphene Networks for High-Performance Anode Material in Lithium-Ion Batteries. Small 2013, 9, 3433–3438. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, L.; Zhang, C.; Casillas, G.; Sun, Z.; Yan, Z.; Ruan, G.; Peng, Z.; Raji, A.-R.O.; Kittrell, C. A seamless three-dimensional carbon nanotube graphene hybrid material. Nat. Commun. 2012, 3, 1225. [Google Scholar] [CrossRef]
- Chang, Y.H.; Lin, C.T.; Chen, T.Y.; Hsu, C.L.; Lee, Y.H.; Zhang, W.J.; Wei, K.H.; Li, L.J. Highly Efficient Electrocatalytic Hydrogen Production by MoSx Grown on Graphene-Protected 3D Ni Foams. Adv. Mater. 2013, 25, 756–760. [Google Scholar] [CrossRef]
- Li, K.H.; Zhang, Z.F.; Li, D.P.; Zhang, W.S.; Yu, X.Q.; Liu, W.; Gong, C.C.; Wei, G.; Su, Z.Q. Biomimetic Ultralight, Highly Porous, Shape-Adjustable, and Biocompatible 3D Graphene Minerals via Incorporation of Self-Assembled Peptide Nanosheets. Adv. Funct. Mater. 2018, 28, 1801056. [Google Scholar] [CrossRef]
- He, Y.M.; Chen, W.J.; Li, X.D.; Zhang, Z.X.; Fu, J.C.; Zhao, C.H.; Xie, E.Q. Freestanding Three-Dimensional Graphene/MnO2 Composite Networks As Ultra light and Flexible Supercapacitor Electrodes. ACS Nano 2013, 7, 174–182. [Google Scholar] [CrossRef]
- Cong, H.-P.; Ren, X.-C.; Wang, P.; Yu, S.-H. Macroscopic multifunctional graphene-based hydrogels and aerogels by a metal ion induced self-assembly process. ACS Nano 2012, 6, 2693–2703. [Google Scholar] [CrossRef]
- Vanmaekelbergh, D. Self-assembly of colloidal nanocrystals as route to novel classes of nanostructured materials. Nano Today 2011, 6, 419–437. [Google Scholar] [CrossRef]
- Jiang, X.; Ma, Y.; Li, J.; Fan, Q.; Huang, W. Self-assembly of reduced graphene oxide into three-dimensional architecture by divalent ion linkage. J. Phys. Chem. C 2010, 114, 22462–22465. [Google Scholar] [CrossRef]
- Chen, W.; Yan, L. In situ self-assembly of mild chemical reduction graphene for three-dimensional architectures. Nanoscale 2011, 3, 3132–3137. [Google Scholar] [CrossRef]
- Ji, C.-C.; Xu, M.-W.; Bao, S.-J.; Cai, C.-J.; Lu, Z.-J.; Chai, H.; Yang, F.; Wei, H. Self-assembly of three-dimensional interconnected graphene-based aerogels and its application in supercapacitors. J. Colloid Interface Sci. 2013, 407, 416–424. [Google Scholar] [CrossRef]
- Ye, S.B.; Feng, J.C. Self-Assembled Three-Dimensional Hierarchical Graphene/Polypyrrole Nanotube Hybrid Aerogel and Its Application for Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 9671–9679. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, H.; Chen, P.; Nie, L.H.; Li, C.H.; Li, S.K. Self-assembled three-dimensional hierarchical graphene hybrid hydrogels with ultrathin beta-MnO2 nanobelts for high performance supercapacitors. J. Mater. Chem. A 2015, 3, 1540–1548. [Google Scholar] [CrossRef]
- Wu, C.; Huang, X.Y.; Wang, G.L.; Lv, L.B.; Chen, G.; Li, G.Y.; Jiang, P.K. Highly Conductive Nanocomposites with Three-Dimensional, Compactly Interconnected Graphene Networks via a Self-Assembly Process. Adv. Funct. Mater. 2013, 23, 506–513. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Lin, C.W.; Zhu, G.; Marsh, K.L.; Hwang, J.Y.; Zhang, Q.; Li, Y.; Wang, H.; Kaner, R.B. 3D freeze-casting of cellular graphene films for ultrahigh-power-density supercapacitors. Adv. Mater. 2016, 28, 6719–6726. [Google Scholar] [CrossRef]
- Han, Z.; Tang, Z.H.; Li, P.; Yang, G.Z.; Zheng, Q.B.; Yang, J.H. Ammonia solution strengthened three-dimensional macro-porous graphene aerogel. Nanoscale 2013, 5, 5462–5467. [Google Scholar] [CrossRef]
- Zhang, R.J.; Cao, Y.C.; Li, P.X.; Zang, X.B.; Sun, P.Z.; Wang, K.L.; Zhong, M.L.; Wei, J.Q.; Wu, D.H.; Kang, F.Y.; et al. Three-dimensional porous graphene sponges assembled with the combination of surfactant and freeze-drying. Nano Res. 2014, 7, 1477–1487. [Google Scholar] [CrossRef]
- Vickery, J.L.; Patil, A.J.; Mann, S. Fabrication of Graphene-Polymer Nanocomposites with Higher-Order Three-Dimensional Architectures. Adv. Mater. 2009, 21, 2180–2184. [Google Scholar] [CrossRef]
- Lu, S.T.; Chen, Y.; Wu, X.H.; Wang, Z.D.; Li, Y. Three-Dimensional Sulfur/Graphene Multifunctional Hybrid Sponges for Lithium-Sulfur Batteries with Large Areal Mass Loading. Sci. Rep. 2014, 4, 4629. [Google Scholar] [CrossRef]
- Mohandes, F.; Salavati-Niasari, M. Freeze-drying synthesis, characterization and in vitro bioactivity of chitosan/graphene oxide/hydroxyapatite nanocomposite. RSC Adv. 2014, 4, 25993–26001. [Google Scholar] [CrossRef]
- Foster, C.W.; Down, M.P.; Zhang, Y.; Ji, X.; Rowley-Neale, S.J.; Smith, G.C.; Kelly, P.J.; Banks, C.E. 3D printed graphene based energy storage devices. Sci. Rep. 2017, 7, 42233. [Google Scholar] [CrossRef]
- Zhu, C.; Han, T.Y.J.; Duoss, E.B.; Golobic, A.M.; Kuntz, J.D.; Spadaccini, C.M.; Worsley, M.A. Highly compressible 3D periodic graphene aerogel microlattices. Nat. Commun. 2015, 6, 6962. [Google Scholar] [CrossRef]
- Jakus, A.E.; Secor, E.B.; Rutz, A.L.; Jordan, S.W.; Hersam, M.C.; Shah, R.N. Three-Dimensional Printing of High-Content Graphene Scaffolds for Electronic and Biomedical Applications. ACS Nano 2015, 9, 4636–4648. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, T.Y.; Qian, F.; Han, T.Y.J.; Duoss, E.B.; Kuntz, J.D.; Spadaccini, C.M.; Worsley, M.A.; Li, Y. Supercapacitors Based on Three-Dimensional Hierarchical Graphene Aerogels with Periodic Macropores. Nano Lett. 2016, 16, 3448–3456. [Google Scholar] [CrossRef]
- Xu, C.; Cui, A.; Xu, Y.; Fu, X. Graphene oxide–TiO2 composite filtration membranes and their potential application for water purification. Carbon 2013, 62, 465–471. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W.; Xu, N. Graphene-based membranes. Chem. Soc. Rev. 2015, 44, 5016–5030. [Google Scholar] [CrossRef]
- Cao, K.; Jiang, Z.; Zhao, J.; Zhao, C.; Gao, C.; Pan, F.; Wang, B.; Cao, X.; Yang, J. Enhanced water permeation through sodium alginate membranes by incorporating graphene oxides. J. Membr. Sci. 2014, 469, 272–283. [Google Scholar] [CrossRef]
- Zarrin, H.; Higgins, D.; Jun, Y.; Chen, Z.; Fowler, M. Functionalized graphene oxide nanocomposite membrane for low humidity and high temperature proton exchange membrane fuel cells. J. Phys. Chem. C 2011, 115, 20774–20781. [Google Scholar] [CrossRef]
- Sreeprasad, T.; Maliyekkal, S.M.; Lisha, K.; Pradeep, T. Reduced graphene oxide-metal/metal oxide composites: Facile synthesis and application in water purification. J. Hazard. Mater. 2011, 186, 921–931. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, P.; Chen, Y.; Su, Z.; Wei, G. Recent advances in the fabrication and structure-specific applications of graphene-based inorganic hybrid membranes. Nanoscale 2015, 7, 5080–5093. [Google Scholar] [CrossRef]
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Xia, J.; Zhang, F.; Li, F.; Xia, Y.; Li, Y. Novel GO-blended PVDF ultrafiltration membranes. Desalination 2012, 299, 50–54. [Google Scholar] [CrossRef]
- Cao, Y.-C.; Xu, C.; Wu, X.; Wang, X.; Xing, L.; Scott, K. A poly (ethylene oxide)/graphene oxide electrolyte membrane for low temperature polymer fuel cells. J. Power Sources 2011, 196, 8377–8382. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, D.; Cho, M.; Lee, S.S.; Zhang, F.; Biswas, P.; Fortner, J.D. In situ photocatalytic synthesis of Ag nanoparticles (nAg) by crumpled graphene oxide composite membranes for filtration and disinfection applications. Environ. Sci. Technol. 2016, 50, 2514–2521. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, W.-N.; Liu, D.; Nie, Y.; Li, W.; Wu, J.; Zhang, F.; Biswas, P.; Fortner, J.D. Engineered crumpled graphene oxide nanocomposite membrane assemblies for advanced water treatment processes. Environ. Sci. Technol. 2015, 49, 6846–6854. [Google Scholar] [CrossRef]
- Faust, S.D.; Aly, O.M. Chemistry of Water Treatment; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Kyzas, G.Z.; Deliyanni, E.A.; Matis, K.A. Graphene oxide and its application as an adsorbent for wastewater treatment. J. Chem. Technol. Biotechnol. 2014, 89, 196–205. [Google Scholar] [CrossRef]
- Bi, H.; Yin, K.; Xie, X.; Zhou, Y.; Wan, N.; Xu, F.; Banhart, F.; Sun, L.; Ruoff, R.S. Low temperature casting of graphene with high compressive strength. Adv. Mater. 2012, 24, 5124–5129. [Google Scholar] [CrossRef]
- Worsley, M.A.; Olson, T.Y.; Lee, J.R.; Willey, T.M.; Nielsen, M.H.; Roberts, S.K.; Pauzauskie, P.J.; Biener, J.; Satcher, J.H., Jr.; Baumann, T.F. High surface area, sp2-cross-linked three-dimensional graphene monoliths. J. Phys. Chem. Lett. 2011, 2, 921–925. [Google Scholar] [CrossRef]
- Guo, L.; Ye, P.; Wang, J.; Fu, F.; Wu, Z. Three-dimensional Fe3O4-graphene macroscopic composites for arsenic and arsenate removal. J. Hazard. Mater. 2015, 298, 28–35. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Ang, H.-M.; Tadé, M. Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials. Chem. Eng. J. 2013, 226, 336–347. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Q.; Sun, Y.; Bai, H.; Shi, G. Three-dimensional self-assembly of graphene oxide and DNA into multifunctional hydrogels. ACS Nano 2010, 4, 7358–7362. [Google Scholar] [CrossRef]
- Bi, H.; Xie, X.; Yin, K.; Zhou, Y.; Wan, S.; He, L.; Xu, F.; Banhart, F.; Sun, L.; Ruoff, R.S. Spongy graphene as a highly efficient and recyclable sorbent for oils and organic solvents. Adv. Funct. Mater. 2012, 22, 4421–4425. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Wang, Y.; Yu, W.; Chan, H.L. Microfluidic reactors for photocatalytic water purification. Lab Chip 2014, 14, 1074–1082. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.L.; Liu, R.-S.; Tsai, D.P. Plasmonic photocatalysis. Rep. Prog. Phys. 2013, 76, 046401. [Google Scholar] [CrossRef] [Green Version]
- Lettmann, C.; Hinrichs, H.; Maier, W.F. Combinatorial discovery of new photocatalysts for water purification with visible light. Angew. Chem. Int. Ed. 2001, 40, 3160–3164. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Umar, M.; Aziz, H.A. Photocatalytic degradation of organic pollutants in water. In Organic Pollutants-Monitoring, Risk and Treatment; IntechOpen: London, UK, 2013. [Google Scholar]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Kamaei, M.; Rashedi, H.; Dastgheib, S.; Tasharrofi, S. Comparing photocatalytic degradation of gaseous ethylbenzene using N-doped and pure TiO2 nano-catalysts coated on glass beads under both UV and visible light irradiation. Catalysts 2018, 8, 466. [Google Scholar] [CrossRef]
- Serpone, N. Relative photonic efficiencies and quantum yields in heterogeneous photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 104, 1–12. [Google Scholar] [CrossRef]
- Jiménez, S.; Micó, M.; Arnaldos, M.; Medina, F.; Contreras, S. State of the art of produced water treatment. Chemosphere 2018, 192, 186–208. [Google Scholar] [CrossRef]
- Zhang, K.; Kemp, K.C.; Chandra, V. Homogeneous anchoring of TiO2 nanoparticles on graphene sheets for waste water treatment. Mater. Lett. 2012, 81, 127–130. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Bai, H.; Li, L. Graphene oxide–chitosan composite hydrogels as broad-spectrum adsorbents for water purification. J. Mater. Chem. A 2013, 1, 1992–2001. [Google Scholar] [CrossRef]
- Bhanvase, B.; Shende, T.; Sonawane, S. A review on graphene-TiO2 and doped graphene-TiO2 nanocomposite photocatalyst for water and wastewater treatment. Environ. Technol. Rev. 2017, 6, 1–14. [Google Scholar] [CrossRef]
- Szabó, T.; Veres, Á.; Cho, E.; Khim, J.; Varga, N.; Dékány, I. Photocatalyst separation from aqueous dispersion using graphene oxide/TiO2 nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2013, 433, 230–239. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, W.-N.; Biswas, P.; Fortner, J.D. Facile aerosol synthesis and characterization of ternary crumpled graphene-TiO2-magnetite nanocomposites for advanced water treatment. ACS Appl. Mater. Interfaces 2014, 6, 11766–11774. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, K. Reduced graphene oxide-TiO2 nanocomposite with high photocatalystic activity for the degradation of rhodamine B. J. Mol. Catal. A Chem. 2011, 345, 101–107. [Google Scholar] [CrossRef]
- Jiang, G.; Lin, Z.; Chen, C.; Zhu, L.; Chang, Q.; Wang, N.; Wei, W.; Tang, H. TiO2 nanoparticles assembled on graphene oxide nanosheets with high photocatalytic activity for removal of pollutants. Carbon 2011, 49, 2693–2701. [Google Scholar] [CrossRef]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef]
- Yoo, D.-H.; Cuong, T.V.; Pham, V.H.; Chung, J.S.; Khoa, N.T.; Kim, E.J.; Hahn, S.H. Enhanced photocatalytic activity of graphene oxide decorated on TiO2 films under UV and visible irradiation. Curr. Appl. Phys. 2011, 11, 805–808. [Google Scholar] [CrossRef]
- Stengl, V.; Popelková, D.; Vlácil, P. TiO2–graphene nanocomposite as high performace photocatalysts. J. Phys. Chem. C 2011, 115, 25209–25218. [Google Scholar] [CrossRef]
- Rajeshwar, K.; Ibanez, J.; Swain, G. Electrochemistry and the environment. J. Appl. Electrochem. 1994, 24, 1077–1091. [Google Scholar] [CrossRef]
- Santos, I.D.; Dezotti, M.; Dutra, A.J. Electrochemical treatment of effluents from petroleum industry using a Ti/RuO2 anode. Chem. Eng. J. 2013, 226, 293–299. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Electrocatalytic materials for the electrochemical oxidation of synthetic dyes. Appl. Catal. B Environ. 2007, 75, 95–101. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Lee, S.; Kim, D.; Hwang, W.; Wang, Z.L. Hybrid energy cell for degradation of methyl orange by self-powered electrocatalytic oxidation. Nano Lett. 2013, 13, 803–808. [Google Scholar] [CrossRef]
- Zhou, L.; Deng, H.; Wan, J.; Shi, J.; Su, T. A solvothermal method to produce RGO-Fe3O4 hybrid composite for fast chromium removal from aqueous solution. Appl. Surf. Sci. 2013, 283, 1024–1031. [Google Scholar] [CrossRef]
- Canizares, P.; Martınez, F.; Dıaz, M.; Garcıa-Gómez, J.; Rodrigo, M. Electrochemical oxidation of aqueous phenol wastes using active and nonactive electrodes. J. Electrochem. Soc. 2002, 149, D118–D124. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, M.; Lu, F. Electrochemical quartz crystal microbalance investigation of surface fouling due to phenol oxidation. J. Electroanal. Chem. 1998, 444, 127–132. [Google Scholar] [CrossRef]
- Zhou, M.; Dai, Q.; Lei, L.; Ma, C.a.; Wang, D. Long life modified lead dioxide anode for organic wastewater treatment: Electrochemical characteristics and degradation mechanism. Environ. Sci. Technol. 2005, 39, 363–370. [Google Scholar] [CrossRef]
- Zhou, M.; Wu, Z.; Wang, D. Electrocatalytic degradation of phenol in acidic and saline wastewater. J. Environ. Sci. Health Part A 2002, 37, 1263–1275. [Google Scholar] [CrossRef]
- Kim, S.; Choi, S.K.; Yoon, B.Y.; Lim, S.K.; Park, H. Effects of electrolyte on the electrocatalytic activities of RuO2/Ti and Sb-SnO2/Ti anodes for water treatment. Appl. Catal. B Environ. 2010, 97, 135–141. [Google Scholar] [CrossRef]
- Ma, H.; Zhuo, Q.; Wang, B. Electro-catalytic degradation of methylene blue wastewater assisted by Fe2O3-modified kaolin. Chem. Eng. J. 2009, 155, 248–253. [Google Scholar] [CrossRef]
- Lv, G.; Wu, D.; Fu, R. Performance of carbon aerogels particle electrodes for the aqueous phase electro-catalytic oxidation of simulated phenol wastewaters. J. Hazard. Mater. 2009, 165, 961–966. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Maldonado-Hódar, F. Carbon aerogels for catalysis applications: An overview. Carbon 2005, 43, 455–465. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, C.; Liu, F.; Hou, Y. Hybrid of Iron Nitride and Nitrogen-Doped Graphene Aerogel as Synergistic Catalyst for Oxygen Reduction Reaction. Adv. Funct. Mater. 2014, 24, 2930–2937. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Zhou, L.; Zhu, H.; Wan, F.; Wang, Y.; Zhang, D. Performance of nitrogen-doped graphene aerogel particle electrodes for electro-catalytic oxidation of simulated Bisphenol A wastewaters. J. Hazard. Mater. 2017, 332, 70–78. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, Y.; Yang, F. FeOOH-catalyzed heterogeneous electro-Fenton system upon anthraquinone@ graphene nanohybrid cathode in a divided electrolytic cell: Catholyte-regulated catalytic oxidation performance and mechanism. J. Electrochem. Soc. 2015, 162, H357–H365. [Google Scholar] [CrossRef]
- Zhai, C.; Zhu, M.; Ren, F.; Yao, Z.; Du, Y.; Yang, P. Enhanced photoelectrocatalytic performance of titanium dioxide/carbon cloth based photoelectrodes by graphene modification under visible-light irradiation. J. Hazard. Mater. 2013, 263, 291–298. [Google Scholar] [CrossRef]
- Xiong, B.; Zhou, Y.; Zhao, Y.; Wang, J.; Chen, X.; O’Hayre, R.; Shao, Z. The use of nitrogen-doped graphene supporting Pt nanoparticles as a catalyst for methanol electrocatalytic oxidation. Carbon 2013, 52, 181–192. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, W.; An, W.; Liu, L.; Liang, Y.; Zhu, Y. Combination of photoelectrocatalysis and adsorption for removal of bisphenol A over TiO2-graphene hydrogel with 3D network structure. Appl. Catal. B Environ. 2018, 221, 36–46. [Google Scholar] [CrossRef]
- Duan, T.; Wen, Q.; Chen, Y.; Zhou, Y.; Duan, Y. Enhancing electrocatalytic performance of Sb-doped SnO2 electrode by compositing nitrogen-doped graphene nanosheets. J. Hazard. Mater. 2014, 280, 304–314. [Google Scholar] [CrossRef]
- Alexander, M. Biodegradation and Bioremediation; Gulf Professional Publishing: Oxford, UK, 1999. [Google Scholar]
- Marinescu, M.; Dumitru, M.; Lăcătuşu, A.R. Biodegradation of petroleum hydrocarbons in an artificial polluted soil. Res. J. Agric. Sci. 2009, 41, 157–162. [Google Scholar]
- Rajasulochana, P.; Preethy, V. Comparison on efficiency of various techniques in treatment of waste and sewage water–A comprehensive review. Resour. Effic. Technol. 2016, 2, 175–184. [Google Scholar] [CrossRef]
- Joutey, N.T.; Bahafid, W.; Sayel, H.; El Ghachtouli, N. Biodegradation: Involved microorganisms and genetically engineered microorganisms. In Biodegradation-Life of Science; InTechOpen: London, UK, 2013. [Google Scholar]
- Hatzinger, P.B. Perchlorate Biodegradation for Water Treatment; ACS Publications: Washington, DC, USA, 2005. [Google Scholar]
- Bernhard, M.; Müller, J.; Knepper, T.P. Biodegradation of persistent polar pollutants in wastewater: Comparison of an optimised lab-scale membrane bioreactor and activated sludge treatment. Water Res. 2006, 40, 3419–3428. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Efficient simultaneous adsorption-biodegradation of high-concentrated N, N-dimethylformamide from water by Paracoccus denitrificans-graphene oxide microcomposites. Sci. Rep. 2016, 6, 20003. [Google Scholar] [CrossRef]
- Vineh, M.B.; Saboury, A.A.; Poostchi, A.A.; Rashidi, A.M.; Parivar, K. Stability and activity improvement of horseradish peroxidase by covalent immobilization on functionalized reduced graphene oxide and biodegradation of high phenol concentration. Int. J. Biol. Macromol. 2018, 106, 1314–1322. [Google Scholar] [CrossRef]
- Suss, M.; Porada, S.; Sun, X.; Biesheuvel, P.; Yoon, J.; Presser, V. Water desalination via capacitive deionization: What is it and what can we expect from it? Energy Environ. Sci. 2015, 8, 2296–2319. [Google Scholar] [CrossRef]
- He, F.; Biesheuvel, P.; Bazant, M.Z.; Hatton, T.A. Theory of water treatment by capacitive deionization with redox active porous electrodes. Water Res. 2018, 132, 282–291. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; Bao, S.; Song, S. Desalination by capacitive deionization with carbon-based materials as electrode: A review. Surf. Rev. Lett. 2013, 20, 1330003. [Google Scholar] [CrossRef]
- Zhang, C.; He, D.; Ma, J.; Tang, W.; Waite, T.D. Faradaic reactions in capacitive deionization (CDI)-problems and possibilities: A review. Water Res. 2018, 128, 314–330. [Google Scholar] [CrossRef]
- Porada, S.; Zhao, R.; Van Der Wal, A.; Presser, V.; Biesheuvel, P. Review on the science and technology of water desalination by capacitive deionization. Prog. Mater Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef] [Green Version]
- Villar, I.; Roldan, S.; Ruiz, V.; Granda, M.; Blanco, C.; Menéndez, R.; Santamaría, R. Capacitive deionization of NaCl solutions with modified activated carbon electrodes. Energy Fuels 2010, 24, 3329–3333. [Google Scholar] [CrossRef]
- Nie, C.; Pan, L.; Li, H.; Chen, T.; Lu, T.; Sun, Z. Electrophoretic deposition of carbon nanotubes film electrodes for capacitive deionization. J. Electroanal. Chem. 2012, 666, 85–88. [Google Scholar] [CrossRef]
- Mayes, R.T.; Tsouris, C.; Kiggans, J.O., Jr.; Mahurin, S.M.; DePaoli, D.W.; Dai, S. Hierarchical ordered mesoporous carbon from phloroglucinol-glyoxal and its application in capacitive deionization of brackish water. J. Mater. Chem. 2010, 20, 8674–8678. [Google Scholar] [CrossRef]
- Li, H.; Lu, T.; Pan, L.; Zhang, Y.; Sun, Z. Electrosorption behavior of graphene in NaCl solutions. J. Mater. Chem. 2009, 19, 6773–6779. [Google Scholar] [CrossRef]
- Jia, B.; Zou, L. Graphene nanosheets reduced by a multi-step process as high-performance electrode material for capacitive deionisation. Carbon 2012, 50, 2315–2321. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, Z.-H.; Yu, X.-L.; Kang, F. Graphene oxide-embedded porous carbon nanofiber webs by electrospinning for capacitive deionization. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Nie, C.; Liu, X.; Xu, X.; Sun, Z.; Pan, L. Review on carbon-based composite materials for capacitive deionization. RSC Adv. 2015, 5, 15205–15225. [Google Scholar] [CrossRef]
- Li, H.; Pan, L.; Nie, C.; Liu, Y.; Sun, Z. Reduced graphene oxide and activated carbon composites for capacitive deionization. J. Mater. Chem. 2012, 22, 15556–15561. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, T.; Shi, L.; Peng, Z.; Wen, X.; Zhang, J. Enhanced capacitive deionization performance of graphene/carbon nanotube composites. J. Mater. Chem. 2012, 22, 14696–14704. [Google Scholar] [CrossRef]
- Wimalasiri, Y.; Zou, L. Carbon nanotube/graphene composite for enhanced capacitive deionization performance. Carbon 2013, 59, 464–471. [Google Scholar] [CrossRef]
- Sui, Z.; Meng, Q.; Zhang, X.; Ma, R.; Cao, B. Green synthesis of carbon nanotube-graphene hybrid aerogels and their use as versatile agents for water purification. J. Mater. Chem. 2012, 22, 8767–8771. [Google Scholar] [CrossRef]
- Zhang, D.; Wen, X.; Shi, L.; Yan, T.; Zhang, J. Enhanced capacitive deionization of graphene/mesoporous carbon composites. Nanoscale 2012, 4, 5440–5446. [Google Scholar] [CrossRef]
- Yu, J.-G.; Yu, L.-Y.; Yang, H.; Liu, Q.; Chen, X.-H.; Jiang, X.-Y.; Chen, X.-Q.; Jiao, F.-P. Graphene nanosheets as novel adsorbents in adsorption, preconcentration and removal of gases, organic compounds and metal ions. Sci. Total Environ. 2015, 502, 70–79. [Google Scholar] [CrossRef]
- Liu, M.; Chen, C.; Hu, J.; Wu, X.; Wang, X. Synthesis of magnetite/graphene oxide composite and application for cobalt (II) removal. J. Phys. Chem. C 2011, 115, 25234–25240. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Yang, J.-W. Self-assembled flower-like TiO2 on exfoliated graphite oxide for heavy metal removal. J. Ind. Eng. Chem. 2012, 18, 1178–1185. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, D.; Chen, C.; Wang, X. Enhanced photo-reduction and removal of Cr (VI) on reduced graphene oxide decorated with TiO2 nanoparticles. J. Colloid Interface Sci. 2013, 405, 211–217. [Google Scholar] [CrossRef]
- Tan, L.; Wang, Y.; Liu, Q.; Wang, J.; Jing, X.; Liu, L.; Liu, J.; Song, D. Enhanced adsorption of uranium (VI) using a three-dimensional layered double hydroxide/graphene hybrid material. Chem. Eng. J. 2015, 259, 752–760. [Google Scholar] [CrossRef]
- Kabiri, S.; Tran, D.N.; Cole, M.A.; Losic, D. Functionalized three-dimensional (3D) graphene composite for high efficiency removal of mercury. Environ. Sci. Water Res. Technol. 2016, 2, 390–402. [Google Scholar] [CrossRef]
- Smith, S.C.; Rodrigues, D.F. Carbon-based nanomaterials for removal of chemical and biological contaminants from water: A review of mechanisms and applications. Carbon 2015, 91, 122–143. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Chen, C.; Zhao, G.; Yang, X.; Li, J.; Wang, X. Removal of Cu (II) and fulvic acid by graphene oxide nanosheets decorated with Fe3O4 nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 4991–5000. [Google Scholar] [CrossRef]
- Zong, P.; Wang, S.; Zhao, Y.; Wang, H.; Pan, H.; He, C. Synthesis and application of magnetic graphene/iron oxides composite for the removal of U (VI) from aqueous solutions. Chem. Eng. J. 2013, 220, 45–52. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, F.; Luo, Y.; Zhang, L. Three-dimensional magnetic graphene oxide foam/Fe3O4 nanocomposite as an efficient absorbent for Cr (VI) removal. J. Mater. Sci. 2014, 49, 4236–4245. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, X.; Zhang, Y.; Gu, W.; Li, B.; Xian, Y. Synthesis of water-soluble magnetic graphene nanocomposites for recyclable removal of heavy metal ions. J. Mater. Chem. A 2013, 1, 1745–1753. [Google Scholar] [CrossRef]
- Li, L.; Zhou, G.; Weng, Z.; Shan, X.-Y.; Li, F.; Cheng, H.-M. Monolithic Fe2O3/graphene hybrid for highly efficient lithium storage and arsenic removal. Carbon 2014, 67, 500–507. [Google Scholar] [CrossRef]
- Cao, Y.; Li, X. Adsorption of graphene for the removal of inorganic pollutants in water purification: A review. Adsorption 2014, 20, 713–727. [Google Scholar] [CrossRef]
- Reyes Bahena, J.; Robledo Cabrera, A.; López Valdivieso, A.; Herrera Urbina, R. Fluoride adsorption onto α-Al2O3 and its effect on the zeta potential at the alumina-aqueous electrolyte interface. Sep. Sci. Technol. 2002, 37, 1973–1987. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, Y.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene-polypyrrole nanocomposite as a highly efficient and low cost electrically switched ion exchanger for removing ClO4-from wastewater. ACS Appl. Mater. Interfaces 2011, 3, 3633–3637. [Google Scholar] [CrossRef]
- Ramesha, G.; Kumara, A.V.; Muralidhara, H.; Sampath, S. Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes. J. Colloid Interface Sci. 2011, 361, 270–277. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Ye, X.; Hu, K.; Zhong, H.; Yu, J.; Jin, M.; Guo, Z. A facile one-step approach to functionalized graphene oxide-based hydrogels used as effective adsorbents toward anionic dyes. Appl. Surf. Sci. 2014, 308, 82–90. [Google Scholar] [CrossRef]
- Janoš, P. Sorption of basic dyes onto iron humate. Environ. Sci. Technol. 2003, 37, 5792–5798. [Google Scholar] [CrossRef]
- Natarajan, S.; Bajaj, H.C.; Tayade, R.J. Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process. J. Environ. Sci. 2018, 65, 201–222. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef]
- Khataee, A.; Kasiri, M.B. Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide: Influence of the chemical structure of dyes. J. Mol. Catal. A Chem. 2010, 328, 8–26. [Google Scholar] [CrossRef]
- Khan, R.; Bhawana, P.; Fulekar, M. Microbial decolorization and degradation of synthetic dyes: A review. Rev. Environ. Sci. Bio/Technol. 2013, 12, 75–97. [Google Scholar] [CrossRef]
- Shittu, I.; Edathil, A.A.; Alsaeedi, A.; Al-Asheh, S.; Polychronopoulou, K.; Banat, F. Development of novel surfactant functionalized porous graphitic carbon as an efficient adsorbent for the removal of methylene blue dye from aqueous solutions. J. Water Process Eng. 2019, 28, 69–81. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kumar, R.; Nayak, A.; Saleh, T.A.; Barakat, M. Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: A review. Adv. Colloid Interface Sci. 2013, 193, 24–34. [Google Scholar] [CrossRef]
- Liu, F.; Chung, S.; Oh, G.; Seo, T.S. Three-dimensional graphene oxide nanostructure for fast and efficient water-soluble dye removal. ACS Appl. Mater. Interfaces 2012, 4, 922–927. [Google Scholar] [CrossRef]
- He, K.; Chen, G.; Zeng, G.; Chen, A.; Huang, Z.; Shi, J.; Huang, T.; Peng, M.; Hu, L. Three-dimensional graphene supported catalysts for organic dyes degradation. Appl. Catal. B Environ. 2018, 228, 19–28. [Google Scholar] [CrossRef]
- Mei, J.-Y.; Qi, P.; Wei, X.-N.; Zheng, X.-C.; Wang, Q.; Guan, X.-X. Assembly and enhanced elimination performance of 3D graphene aerogel-zinc oxide hybrids for methylene blue dye in water. Mater. Res. Bull. 2019, 109, 141–148. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, D.; Zhu, Y.; Li, Z.; Li, Z.; Tian, H.; Liu, H. Graphene oxide/chitin nanofibril composite foams as column adsorbents for aqueous pollutants. Carbohydr. Polym. 2016, 144, 230–237. [Google Scholar] [CrossRef]
- Shi, Y.-C.; Wang, A.-J.; Wu, X.-L.; Chen, J.-R.; Feng, J.-J. Green-assembly of three-dimensional porous graphene hydrogels for efficient removal of organic dyes. J. Colloid Interface Sci. 2016, 484, 254–262. [Google Scholar] [CrossRef]
- Yusuf, M.; Khan, M.A.; Otero, M.; Abdullah, E.; Hosomi, M.; Terada, A.; Riya, S. Synthesis of CTAB intercalated graphene and its application for the adsorption of AR265 and AO7 dyes from water. J. Colloid Interface Sci. 2017, 493, 51–61. [Google Scholar] [CrossRef]
- Liu, S.; Yao, F.; Oderinde, O.; Zhang, Z.; Fu, G. Green synthesis of oriented xanthan gum–graphene oxide hybrid aerogels for water purification. Carbohydr. Polym. 2017, 174, 392–399. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Fu, J.; Lazaridis, N.K.; Bikiaris, D.N.; Matis, K.A. New approaches on the removal of pharmaceuticals from wastewaters with adsorbent materials. J. Mol. Liq. 2015, 209, 87–93. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment-a review-part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bikiaris, D.N.; Seredych, M.; Bandosz, T.J.; Deliyanni, E.A. Removal of dorzolamide from biomedical wastewaters with adsorption onto graphite oxide/poly (acrylic acid) grafted chitosan nanocomposite. Bioresour. Technol. 2014, 152, 399–406. [Google Scholar] [CrossRef]
- Yang, X.; Chen, C.; Li, J.; Zhao, G.; Ren, X.; Wang, X. Graphene oxide-iron oxide and reduced graphene oxide-iron oxide hybrid materials for the removal of organic and inorganic pollutants. RSC Adv. 2012, 2, 8821–8826. [Google Scholar] [CrossRef]
- Yamaguchi, N.U.; Bergamasco, R.; Hamoudi, S. Magnetic MnFe2O4-graphene hybrid composite for efficient removal of glyphosate from water. Chem. Eng. J. 2016, 295, 391–402. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Lai, X.; Su, X.; Liang, T.; Zeng, X. Thiolated graphene-based superhydrophobic sponges for oil-water separation. Chem. Eng. J. 2017, 316, 736–743. [Google Scholar] [CrossRef]
- Chi, K.; Zhang, Z.; Xi, J.; Huang, Y.; Xiao, F.; Wang, S.; Liu, Y. Freestanding graphene paper supported three-dimensional porous graphene-polyaniline nanocomposite synthesized by inkjet printing and in flexible all-solid-state supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 16312–16319. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, P.; Liu, X.; Yan, B.; Xiang, L.; Zhang, J.; Gong, L.; Huang, J.; Cui, K.; Zhu, L. An amphiphobic graphene-based hydrogel as oil-water separator and oil fence material. Chem. Eng. J. 2018, 353, 708–716. [Google Scholar] [CrossRef]
- Jung, G.S.; Buehler, M.J. Multiscale mechanics of triply periodic minimal surfaces of three-dimensional graphene foams. Nano Lett. 2018, 18, 4845–4853. [Google Scholar] [CrossRef]
- Wang, J.-N.; Zhang, Y.-L.; Liu, Y.; Zheng, W.; Lee, L.P.; Sun, H.-B. Recent developments in superhydrophobic graphene and graphene-related materials: From preparation to potential applications. Nanoscale 2015, 7, 7101–7114. [Google Scholar] [CrossRef]
- Lee, J.-S.; Yoon, J.-C.; Jang, J.-H. A route towards superhydrophobic graphene surfaces: Surface-treated reduced graphene oxide spheres. J. Mater. Chem. A 2013, 1, 7312–7315. [Google Scholar] [CrossRef]
- Gu, J.; Fan, H.; Li, C.; Caro, J.; Meng, H. Robust Superhydrophobic/Superoleophilic Wrinkled Microspherical MOF@ rGO Composites for Efficient Oil-Water Separation. Angew. Chem. 2019, 131, 5351–5355. [Google Scholar] [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef] [Green Version]
- Sadrzadeh, M.; Mohammadi, T. Sea water desalination using electrodialysis. Desalination 2008, 221, 440–447. [Google Scholar] [CrossRef]
- Lv, H.; Wang, Y.; Wu, L.; Hu, Y. Numerical simulation and optimization of the flash chamber for multi-stage flash seawater desalination. Desalination 2019, 465, 69–78. [Google Scholar] [CrossRef]
- Choi, J.; Dorji, P.; Shon, H.K.; Hong, S. Applications of capacitive deionization: Desalination, softening, selective removal, and energy efficiency. Desalination 2019, 449, 118–130. [Google Scholar] [CrossRef]
- Scida, K.; Stege, P.W.; Haby, G.; Messina, G.A.; García, C.D. Recent applications of carbon-based nanomaterials in analytical chemistry: Critical review. Anal. Chim. Acta 2011, 691, 6–17. [Google Scholar] [CrossRef] [Green Version]
- Akin, I.; Zor, E.; Bingol, H.; Ersoz, M. Green synthesis of reduced graphene oxide/polyaniline composite and its application for salt rejection by polysulfone-based composite membranes. J. Phys. Chem. B 2014, 118, 5707–5716. [Google Scholar] [CrossRef]
- Lou, Y.; Liu, G.; Liu, S.; Shen, J.; Jin, W. A facile way to prepare ceramic-supported graphene oxide composite membrane via silane-graft modification. Appl. Surf. Sci. 2014, 307, 631–637. [Google Scholar]
- Hu, M.; Mi, B. Enabling graphene oxide nanosheets as water separation membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. [Google Scholar] [CrossRef]
- Goh, P.; Ismail, A. Graphene-based nanomaterial: The state-of-the-art material for cutting edge desalination technology. Desalination 2015, 356, 115–128. [Google Scholar] [CrossRef]
- Qin, M.; Deshmukh, A.; Epsztein, R.; Patel, S.K.; Owoseni, O.M.; Walker, W.S.; Elimelech, M. Comparison of energy consumption in desalination by capacitive deionization and reverse osmosis. Desalination 2019, 455, 100–114. [Google Scholar] [CrossRef]
- Gupta, S.S.; Islam, M.R.; Pradeep, T. Capacitive Deionization (CDI): An Alternative Cost-Efficient Desalination Technique. In Advances in Water Purification Techniques; Elsevier: Amsterdam, The Netherlands, 2019; pp. 165–202. [Google Scholar]
- Anderson, M.A.; Cudero, A.L.; Palma, J. Capacitive deionization as an electrochemical means of saving energy and delivering clean water. Comparison to present desalination practices: Will it compete? Electrochim. Acta 2010, 55, 3845–3856. [Google Scholar] [CrossRef]
- Yin, H.; Tang, H.; Wang, D.; Gao, Y.; Tang, Z. Facile synthesis of surfactant-free Au cluster/graphene hybrids for high-performance oxygen reduction reaction. ACS Nano 2012, 6, 8288–8297. [Google Scholar] [CrossRef]
- Chen, W.; Li, S.; Chen, C.; Yan, L. Self-assembly and embedding of nanoparticles by in situ reduced graphene for preparation of a 3D graphene/nanoparticle aerogel. Adv. Mater. 2011, 23, 5679–5683. [Google Scholar] [CrossRef]
- Liu, L.; Liao, L.; Meng, Q.; Cao, B. High performance graphene composite microsphere electrodes for capacitive deionisation. Carbon 2015, 90, 75–84. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, S.; Wan, J.; Tang, H.; Chang, L.; He, L.; Zhao, H.; Gao, Y.; Tang, Z. Three-dimensional graphene/metal oxide nanoparticle hybrids for high-performance capacitive deionization of saline water. Adv. Mater. 2013, 25, 6270–6276. [Google Scholar] [CrossRef]
- Nardecchia, S.; Carriazo, D.; Ferrer, M.L.; Gutiérrez, M.C.; del Monte, F. Three dimensional macroporous architectures and aerogels built of carbon nanotubes and/or graphene: Synthesis and applications. Chem. Soc. Rev. 2013, 42, 794–830. [Google Scholar] [CrossRef]
- Tang, H.; Yin, H.; Wang, J.; Yang, N.; Wang, D.; Tang, Z. Molecular Architecture of Cobalt Porphyrin Multilayers on Reduced Graphene Oxide Sheets for High-Performance Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2013, 52, 5585–5589. [Google Scholar] [CrossRef]
- Wang, H.; Mi, X.; Li, Y.; Zhan, S. 3D Graphene-Based Macrostructures for Water Treatment. Adv. Mater. 2019. [Google Scholar] [CrossRef]
- Landon, J.; Gao, X.; Kulengowski, B.; Neathery, J.K.; Liu, K. Impact of pore size characteristics on the electrosorption capacity of carbon xerogel electrodes for capacitive deionization. J. Electrochem. Soc. 2012, 159, A1861–A1866. [Google Scholar] [CrossRef]
- Porada, S.; Weinstein, L.; Dash, R.; Van Der Wal, A.; Bryjak, M.; Gogotsi, Y.; Biesheuvel, P. Water desalination using capacitive deionization with microporous carbon electrodes. ACS Appl. Mater. Interfaces 2012, 4, 1194–1199. [Google Scholar] [CrossRef]
- Leong, Z.Y.; Lu, G.; Yang, H.Y. Three-dimensional graphene oxide and polyvinyl alcohol composites as structured activated carbons for capacitive desalination. Desalination 2019, 451, 172–181. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, D.; Yan, T.; Wen, X.; Zhang, J.; Shi, L.; Zhong, Q. Three-dimensional macroporous graphene architectures as high performance electrodes for capacitive deionization. J. Mater. Chem. A 2013, 1, 11778–11789. [Google Scholar] [CrossRef]
- El-Deen, A.G.; Barakat, N.A.; Kim, H.Y. Graphene wrapped MnO2-nanostructures as effective and stable electrode materials for capacitive deionization desalination technology. Desalination 2014, 344, 289–298. [Google Scholar] [CrossRef]
- Li, H.; Zou, L. Ion-exchange membrane capacitive deionization: A new strategy for brackish water desalination. Desalination 2011, 275, 62–66. [Google Scholar] [CrossRef]
- Amy, G.; Ghaffour, N.; Li, Z.; Francis, L.; Linares, R.V.; Missimer, T.; Lattemann, S. Membrane-based seawater desalination: Present and future prospects. Desalination 2017, 401, 16–21. [Google Scholar] [CrossRef]
- Jeon, S.; Park, H.; Yeo, J.; Yang, S.; Cho, C.H.; Han, M.H.; Kim, D.K. Desalination via a new membrane capacitive deionization process utilizing flow-electrodes. Energy Environ. Sci. 2013, 6, 1471–1475. [Google Scholar] [CrossRef]
- Rommerskirchen, A.; Gendel, Y.; Wessling, M. Single module flow-electrode capacitive deionization for continuous water desalination. Electrochem. Commun. 2015, 60, 34–37. [Google Scholar] [CrossRef]
- Wang, H.; Yan, T.; Liu, P.; Chen, G.; Shi, L.; Zhang, J.; Zhong, Q.; Zhang, D. In situ creating interconnected pores across 3D graphene architectures and their application as high performance electrodes for flow-through deionization capacitors. J. Mater. Chem. A 2016, 4, 4908–4919. [Google Scholar] [CrossRef]
- Liu, P.; Yan, T.; Shi, L.; Park, H.S.; Chen, X.; Zhao, Z.; Zhang, D. Graphene-based materials for capacitive deionization. J. Mater. Chem. A 2017, 5, 13907–13943. [Google Scholar] [CrossRef]
- Huang, Z.-H.; Wang, M.; Wang, L.; Kang, F. Relation between the charge efficiency of activated carbon fiber and its desalination performance. Langmuir 2012, 28, 5079–5084. [Google Scholar] [CrossRef]
- Li, H.; Nie, C.; Pan, L.; Sun, Z. The study of membrane capacitive deionization from charge efficiency. Desalin. Water Treat. 2012, 42, 210–215. [Google Scholar] [CrossRef]









| Adsorbent | Organic Dye | Dye Concentration [mg/L] | Adsorption Performance | Reference |
|---|---|---|---|---|
| 3D GO/DNA composite hydrogels | Safranine O (SO) dye | 100 | Nearly 100% removal efficiency in 24 h; 960 mg/g dye loading capacity | [86] |
| 3D GA-ZnO hybrids | MB dye | 20 | Up to 97.6% removal efficiency in 3 h | [179] |
| 3D GO-CNF composite foams | MB dye | 30 | Up to 98.5% removal efficiency in 4 h | [180] |
| 3D N/S-GHs hydrogels | MB, MG, and CV dyes | 140 | Superior removal efficiency in 10 h; adsorption capacities of 738.1 mg/g for MG, around 625 mg/g for MB and around 600 mg/g for CV | [181] |
| 3D GN-CTAB composites | AO7 and AR265 dyes | 100 | 92.18% and 89.13% removal efficiency for AR265 and AO7, respectively in 3 h; adsorption capacities of 511 mg/g and 356 mg/g for AR265 and AO7, respectively | [182] |
| 3D XG/GO hybrid aerogels | RB and BM dyes | 100 | Around 95% and 96% removal efficiency for RB and MB, respectively in 12 h; adsorption capacities of 244.36 mg/g and 290.57 mg/g for RB and MB, respectively | [183] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Guo, L.; Qi, P.; Liu, X.; Wei, G. Synthesis of Three-Dimensional Graphene-Based Hybrid Materials for Water Purification: A Review. Nanomaterials 2019, 9, 1123. https://doi.org/10.3390/nano9081123
Wang Y, Guo L, Qi P, Liu X, Wei G. Synthesis of Three-Dimensional Graphene-Based Hybrid Materials for Water Purification: A Review. Nanomaterials. 2019; 9(8):1123. https://doi.org/10.3390/nano9081123
Chicago/Turabian StyleWang, Yan, Lei Guo, Pengfei Qi, Xiaomin Liu, and Gang Wei. 2019. "Synthesis of Three-Dimensional Graphene-Based Hybrid Materials for Water Purification: A Review" Nanomaterials 9, no. 8: 1123. https://doi.org/10.3390/nano9081123
APA StyleWang, Y., Guo, L., Qi, P., Liu, X., & Wei, G. (2019). Synthesis of Three-Dimensional Graphene-Based Hybrid Materials for Water Purification: A Review. Nanomaterials, 9(8), 1123. https://doi.org/10.3390/nano9081123