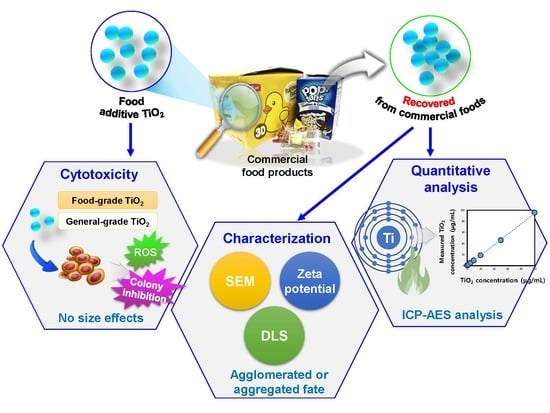
Food Additive Titanium Dioxide and Its Fate in Commercial Foods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Size Fractionation
2.4. Preparation of TiO2-Containing Simulated Foods
2.5. Pretreatment of Simulated Foods, Commercial Products, and Cell Samples for Characterization and Quantitative Analysis
2.6. Quantitative Analysis
2.7. Cell Culture
2.8. Short-Term Cell Proliferation
2.9. Cell Membrane Damage
2.10. Colony-Forming Ability
2.11. Reactive oxygen species (ROS)
2.12. Apoptosis Induction
2.13. Intestinal Transportation
2.14. Statistical Analysis
3. Results
3.1. Characterization of Food Additive TiO2
3.2. Characterization and Quantitative Analysis of TiO2 in Simulated Foods
3.3. Characterization and Quantitative Analysis of TiO2 in Commercial Foods
3.4. Size Fractionation of TiO2
3.5. Inhibition of Cell Proliferation, Membrane Damage, and Colony Formation
3.6. ROS and Apoptosis Induction
3.7. Intestinal Transport Mechanism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- European Food Safety Authority (EFSA). Re-evaluation of titanium dioxide (E 171) as a food additive. EFSA J. 2016, 14, 1–83. [Google Scholar]
- Dudefoi, W.; Moniz, K.; Allen-Vercoe, E.; Ropers, M.H.; Walker, V.K. Impact of food grade and nano-TiO2 particles on a human intestinal community. Food Chem. Toxicol. 2017, 106, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Proquin, H.; Rodríguez-Ibarra, C.; Moonen, C.G.; Urrutia Ortega, I.M.; Briedé, J.J.; de Kok, T.M.; van Loveren, H.; Chirino, Y.I. Titanium dioxide food additive (E171) induces ROS formation and genotoxicity: Contribution of micro and nano-sized fractions. Mutagenesis 2017, 32, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Heringa, M.B.; Geraets, L.; van Eijkeren, J.C.; Vandebriel, R.J.; de Jong, W.H.; Oomen, A.G. Risk assessment of titanium dioxide nanoparticles via oral exposure, including toxicokinetic considerations. Nanotoxicology 2016, 10, 1515–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkler, H.C.; Notter, T.; Meyer, U.; Naegeli, H. Critical review of the safety assessment of titanium dioxide additives in food. J. Nanobiotechnol. 2018, 16, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamat, P.V. Dominance of metal oxides in the era of nanotechnology. J. Phys. Chem. Lett. 2011, 2, 839–840. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=73.575 (accessed on 25 July 2019).
- European Commission. Commission Recommendation of 18 October 2011 on the definition of nanomaterial. Off. J. Eur. Union 2011, 275, 38–40. [Google Scholar]
- Yang, Y.; Doudrick, K.; Bi, X.; Hristovski, K.; Herckes, P.; Westerhoff, P.; Kaegi, R. Characterization of food-grade titanium dioxide: The presence of nanosized particles. Environ. Sci. Technol. 2014, 48, 6391–6400. [Google Scholar] [CrossRef]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef]
- Peters, R.J.B.; van Bemmel, G.; Herrera-Rivera, Z.; Helsper, H.P.F.G.; Marvin, H.J.P.; Weigel, S.; Tromp, P.C.; Oomen, A.G.; Rietveld, A.G.; Bouwmeester, H. Characterization of titanium dioxide nanoparticles in food products: Analytical methods to define nanoparticles. J. Agric. Food. Chem. 2014, 62, 6285–6293. [Google Scholar] [CrossRef]
- Candás-Zapico, S.; Kutscher, D.J.; Montes-Bayón, M.; Bettmer, J. Single particle analysis of TiO2 in candy products using triple quadrupole ICP-MS. Talanta 2018, 180, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Stephan, C.; Westerhoff, P.; Carlander, D.; Duncan, T.V. Measurement methods to detect, characterize, and quantify engineered nanomaterials in foods. Compr. Rev. Food Sci. F. 2014, 13, 693–704. [Google Scholar] [CrossRef]
- Contado, C. Nanomaterials in consumer products: A challenging analytical problem. Front. Chem. 2015, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, A.; Schneider, Y.J. Engineered nanomaterials in food: Implications for food safety and consumer health. Int. J. Env. Res. Public Health 2014, 11, 5720–5750. [Google Scholar] [CrossRef]
- Jo, M.R.; Yu, J.; Kim, H.J.; Song, J.H.; Kim, K.M.; Oh, J.M.; Choi, S.J. Titanium dioxide nanoparticle-biomolecule interactions influence oral absorption. Nanomaterials 2016, 6, 225. [Google Scholar] [CrossRef]
- Go, M.R.; Bae, S.H.; Kim, H.J.; Yu, J.; Choi, S.J. Interactions between food additive silica nanoparticles and food matrices. Front. Microbiol. 2017, 8, 1013. [Google Scholar] [CrossRef]
- Lee, J.A.; Kim, M.K.; Song, J.H.; Jo, M.R.; Yu, J.; Kim, K.M.; Kim, Y.R.; Oh, J.M.; Choi, S.J. Biokinetics of food additive silica nanoparticles and their interactions with food components. Colloids Surf. B 2017, 150, 384–392. [Google Scholar] [CrossRef]
- Cho, W.S.; Kang, B.C.; Lee, J.K.; Jeong, J.; Che, J.H.; Seok, S.H. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part. Fibre Toxicol. 2013, 10, 9. [Google Scholar] [CrossRef]
- Geraets, L.; Oomen, A.G.; Krystek, P.; Jacobsen, N.R.; Wallin, H.; Laurentie, M.; Verharen, H.W.; Brandon, E.F.A.; de Jong, W.H. Tissue distribution and elimination after oral and intravenous administration of different titanium dioxide nanoparticles in rats. Part. Fibre Toxicol. 2014, 11, 30. [Google Scholar] [CrossRef]
- Janer, G.; Mas del Molino, E.; Fernández-Rosas, E.; Fernández, A.; Vázquez-Campos, S. Cell uptake and oral absorption of titanium dioxide nanoparticles. Toxicol. Lett. 2014, 228, 103–110. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, J.A.; Jo, M.R.; Choi, S.J. Bioavailability of silica, titanium dioxide, and zinc oxide nanoparticles in rats. J. Nanosci. Nanotechnol. 2016, 16, 6580–6586. [Google Scholar] [CrossRef] [PubMed]
- Faust, J.J.; Doudrick, K.; Yang, Y.; Westerhoff, P.; Capco, D.G. Food grade titanium dioxide disrupts intestinal brush border microvilli in vitro independent of sedimentation. Cell Biol. Toxicol. 2014, 30, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Coméra, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Taché, S.; Grysan, P.; Reguer, S.; et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 2017, 7, 40373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, X.; Zhou, Y.; Yu, D.; Deng, Y.; Ouyang, J.; Yang, B.; Luo, D.; Zhang, D.; Kuang, H. Gestational exposure to titanium dioxide nanoparticles impairs the placentation through dysregulation of vascularization, proliferation and apoptosis in mice. Int. J. Nanomed. 2018, 13, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Dorier, M.; Béal, D.; Tisseyre, C.; Marie-Desvergne, C.; Dubosson, M.; Barreau, F.; Houdeau, E.; Herlin-Boime, N.; Rabilloud, T.; Carriere, M. The food additive E171 and titanium dioxide nanoparticles indirectly alter the homeostasis of human intestinal epithelial cells in vitro. Environ. Sci. Nano 2019, 6, 1549–1561. [Google Scholar] [CrossRef]
- Kim, N.; Kim, C.; Jung, S.; Park, Y.; Lee, Y.; Jo, J.; Hong, M.; Lee, S.; Oh, Y.; Jung, K. Determination and identification of titanium dioxide nanoparticles in confectionery foods, marketed in South Korea, using inductively coupled plasma optical emission spectrometry and transmission electron microscopy. Food Addit. Contam. A 2018, 35, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- des Rieux, A.; Fievez, V.; Théate, I.; Mast, J.; Préat, V.; Schneider, Y.J. An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells. Eur. J. Pharm. Sci. 2007, 30, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Fenton, H.J.H. Notes on Qualitative Anlaysis, Concise and Explanatory; Cambridge University Press: Cambridge, UK, 1923. [Google Scholar]
- Walker, P.; Tarn, W.H. CRC Handbook of Metal Etchants; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Korn, M.G.A.; Morte, E.S.B.; dos Santos, D.C.M.B.; Castro, J.T.; Barbosa, J.T.P.; Teixeira, A.P.; Fernandes, A.P.; Welz, B.; dos Santos, W.P.C.; dos Santos, E.B.G.N.; et al. Sample preparation for the determination of metals in food samples using spectroanalytical methods—A review. Appl. Spectrosc. Rev. 2008, 43, 67–92. [Google Scholar] [CrossRef]
- Nardi, E.P.; Evangelista, F.S.; Tormen, L.; Saint´Pierre, T.D.; Curtius, A.J.; de Souza, S.S.; Barbosa, F., Jr. The use of inductively coupled plasma mass spectrometry (ICP-MS) for the determination of toxic and essential elements in different types of food samples. Food Chem. 2009, 112, 727–732. [Google Scholar] [CrossRef]
- Ito, S.; Kitamura, T.; Wada, Y.; Yanagida, S. Facile fabrication of mesoporous TiO2 electrodes for dye solar cells: Chemical modification and repetitive coating. Sol. Energy Mater. Sol. Cells 2003, 76, 3–13. [Google Scholar] [CrossRef]
- Park, K.H.; Jin, E.M.; Gu, H.B.; Shim, S.E.; Hong, C.K. Effects of HNO3 treatment of TiO2 nanoparticles on the photovoltaic properties of dye-sensitized solar cells. Mater. Lett. 2009, 63, 2208–2211. [Google Scholar] [CrossRef]
- Bunhu, T.; Kindness, A.; Martincigh, B.S. Determination of titanium dioxide in commercial sunscreens by inductively coupled plasma-optical emission spectrometry. S. Afr. J. Chem. 2011, 64, 139–143. [Google Scholar]
- Dos Santos, E.J.; Dos Santos, M.P.; Herrmann, A.B.; Sturgeon, R.E. Rapid determination of Ti in TiO2 by ICP OES. Anal. Methods-UK 2016, 8, 6463–6467. [Google Scholar] [CrossRef]
- Ekstrand-Hammarström, B.; Akfur, C.M.; Andersson, P.O.; Lejon, C.; Österlund, L.; Bucht, A. Human primary bronchial epithelial cells respond differently to titanium dioxide nanoparticles than the lung epithelial cell lines A549 and BEAS-2B. Nanotoxicology 2012, 6, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Dorier, M.; Brun, E.; Veronesi, G.; Barreau, F.; Pernet-Gallay, K.; Desvergne, C.; Rabilloud, T.; Carapito, C.; Herlin-Boime, N.; Carrière, M. Impact of anatase and rutile titanium dioxide nanoparticles on uptake carriers and efflux pumps in Caco-2 gut epithelial cells. Nanoscale 2015, 11, 7352–7360. [Google Scholar] [CrossRef] [PubMed]
- Dorier, M.; Béal, D.; Marie-Desvergne, C.; Dubosson, M.; Barreau, F.; Houdeau, E.; Herlin-Boime, N.; Carriere, M. Continuous in vitro exposure of intestinal epithelial cells to E171 food additive causes oxidative stress, inducing oxidation of DNA bases but no endoplasmic reticulum stress. Nanotoxicology 2017, 11, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, L.; Zhang, T.; Ren, G.; Yang, Z. Oxidative stress and apoptosis induced by nanosized titanium dioxide in PC12 cells. Toxicology 2010, 267, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, F.; He, J.; Yadav, S.; Wang, H. Titanium dioxide nanoparticles cause apoptosis in BEAS-2B cells through the caspase 8/t-Bid-independent mitochondrial pathway. Toxicol. Lett. 2010, 196, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Rahman, Q.; Kashyap, M.P.; Singh, A.K.; Jain, G.; Jahan, S.; Lohani, M.; Lantow, M.; Pant, A.B. Nano-titanium dioxide induces genotoxicity and apoptosis in human lung cancer cell line, A549. Hum. Exp. Toxicol. 2013, 32, 153–166. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Davoren, M.; Boertz, J.; Schins, R.P.; Hoffmann, E.; Dopp, E. Titanium dioxide nanoparticles induce oxidative stress and DNA-adduct formation but not DNA-breakage in human lung cells. Part. Fibre Toxicol. 2009, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Trouiller, B.; Reliene, R.; Westbrook, A.; Solaimani, P.; Schiestl, R.H. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009, 69, 8784–8789. [Google Scholar] [CrossRef] [PubMed]
- Han, S.G.; Newsome, B.; Hennig, B. Titanium dioxide nanoparticles increase inflammatory responses in vascular endothelial cells. Toxicology 2013, 306, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Sample | Average Size (nm) | Distribution (No. %) | ||
|---|---|---|---|---|
| <100 nm | 100–200 nm | >200 nm | ||
| T1 | 150.10 ± 19.35 b | 1 | 96 | 3 |
| T2 | 165.32 ± 30.68 b | 3 | 80 | 17 |
| T3 | 169.30 ± 29.82 b | 1 | 76 | 23 |
| T4 | 122.49 ± 23.31 b | 12 | 88 | ND |
| T5 | 118.29 ± 25.80 ab | 22 | 74 | 4 |
| Bulk | 130.63 ± 31.07 b | 14 | 84 | 2 |
| Nano | 51.45 ± 11.63 a | 100 | ND | ND |
| Sample | Distribution (No. %) | Z-Average Size (nm) | Zeta Potential (mV) | ||
|---|---|---|---|---|---|
| <100 nm | 100–200 nm | >200 nm | |||
| T1 | ND | 44.73 ± 7.05 | 55.27 ± 7.05 | 285.30 ± 6.68 b | −40.50 ± 3.06 a |
| T2 | ND | 38.97 ± 1.32 | 61.03 ± 1.32 | 299.93 ± 1.79 b | −40.73 ± 2.46 a |
| T3 | ND | 10.43 ± 1.40 | 89.57 ± 1.40 | 345.27 ± 4.45 c | −42.17 ± 1.63 a |
| T4 | ND | 49.97 ± 4.87 | 50.03 ± 4.87 | 291.60 ± 3.76 b | −36.70 ± 2.01 a |
| T5 | ND | 12.53 ± 2.61 | 87.47 ± 2.61 | 340.00 ± 3.94 c | −42.17 ± 3.61 a |
| Bulk | ND | 45.47 ± 1.70 | 54.53 ± 1.70 | 293.10 ± 0.52 b | −37.90 ± 3.38 a |
| Nano | 38.27 ± 18.28 | 61.60 ± 18.10 | 0.13 ± 0.23 | 153.43 ± 11.72 a | 13.77 ± 0.74 b |
| Sample | Distribution (No. %) | Z-Average Size (nm) | Zeta Potential (mV) | |||
|---|---|---|---|---|---|---|
| <100 nm | 100–200 nm | >200 nm | ||||
| T3 | Pristine | ND | 10.43 ± 1.40 | 89.57 ± 1.40 | 345.27 ± 4.45 a | −42.17 ± 1.63 a |
| Sugar powder | ND | 37.03 ± 13.22 | 62.97 ± 13.22 | 327.30 ± 14.90 a | −21.77 ± 6.61 b | |
| Gum | ND | 24.97 ± 3.39 | 75.03 ± 3.39 | 332.30 ± 5.20 a | −17.37 ± 1.99 b | |
| T4 | Pristine | ND | 49.97 ± 4.87 | 50.03 ± 4.87 | 291.60 ± 3.76 a | −36.70 ± 2.04 a |
| Sugar powder | 0.23 ± 0.40 | 36.83 ± 26.04 | 62.93 ± 26.28 | 293.27 ± 16.69 a | −37.67 ± 3.48 a | |
| Gum | ND | 40.07 ± 17.56 | 59.93 ± 17.56 | 315.40 ± 2.46 a | −16.57 ± 1.12 b | |
| Sample | Parameters | TiO2 Concentration (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 5 | 10 | 20 | 50 | 100 | |||
| Pristine | T3 | Recovery (%) | 93.42 ± 2.78 | 93.86 ± 7.43 | 92.14 ± 3.53 | 91.34 ± 1.52 | 90.88 ± 5.72 | 95.12 ± 1.52 |
| LOD (μg/mL) | 0.05 | |||||||
| LOQ (μg/mL) | 0.15 | |||||||
| T4 | Recovery (%) | 102.08 ± 1.67 | 93.31 ± 4.60 | 95.09 ± 4.66 | 91.18 ± 0.57 | 91.88 ± 6.39 | 92.43 ± 2.54 | |
| LOD (μg/mL) | 0.23 | |||||||
| LOQ (μg/mL) | 0.70 | |||||||
| Simulated sugar powder | T3 | Recovery (%) | 96.81 ± 3.48 | 93.19 ± 10.98 | 90.85 ± 8.49 | 92.72 ± 3.49 | 93.65 ± 4.24 | 91.91 ± 2.80 |
| LOD (μg/mL) | 0.34 | |||||||
| LOQ (μg/mL) | 1.04 | |||||||
| T4 | Recovery (%) | 99.15 ± 2.64 | 97.96 ± 3.59 | 92.12 ± 3.96 | 95.01 ± 2.57 | 91.10 ± 7.21 | 93.50 ± 3.67 | |
| LOD (μg/mL) | 0.18 | |||||||
| LOQ (μg/mL) | 0.55 | |||||||
| Simulated gum | T3 | Recovery (%) | 93.96 ± 4.89 | 93.96 ± 3.50 | 93.41 ± 3.01 | 97.72 ± 2.34 | 90.03 ± 5.48 | 93.90 ± 0.64 |
| LOD (μg/mL) | 0.03 | |||||||
| LOQ (μg/mL) | 0.09 | |||||||
| T4 | Recovery (%) | 97.58 ± 3.64 | 96.30 ± 3.98 | 93.74 ± 2.35 | 90.54 ± 1.16 | 94.04 ± 2.82 | 92.81 ± 1.88 | |
| LOD (μg/mL) | 0.21 | |||||||
| LOQ (μg/mL) | 0.62 | |||||||
| Commercial Products | Distribution (No. %) | Z-Average Size (nm) | Zeta Potential (mV) | TiO2 Contents (mg/g) | ||
|---|---|---|---|---|---|---|
| <100 nm | 100–200 nm | >200 nm | ||||
| Candy 1 | ND | ND | 100.00 ± 0.00 | 417.80 ± 25.06 | −35.37 ± 2.03 | 1.09 ± 0.03 |
| Candy 2 | 3.83 ± 6.64 | 39.67 ± 25.52 | 56.50 ± 31.92 | 306.83 ± 59.91 | −13.13 ± 0.45 | 9.87 ± 0.21 |
| Chocolate 1 | 2.10 ± 3.64 | 25.93 ± 37.80 | 71.97 ± 35.75 | 242.53 ± 45.60 | −12.03 ± 2.83 | 8.63 ± 0.04 |
| Chocolate 2 | ND | 27.03 ± 13.11 | 72.97 ± 13.11 | 264.97 ± 14.55 | −31.87 ± 1.50 | 6.32 ± 0.63 |
| Gum | 11.73 ± 16.82 | 47.50 ± 14.81 | 40.77 ± 22.17 | 344.90 ± 4.78 | −14.50 ± 0.62 | 3.53 ± 0.12 |
| Jelly 1 | 0.90 ± 1.56 | 35.47 ± 27.51 | 63.63 ± 28.93 | 363.17 ± 11.29 | −37.23 ± 0.72 | 1.30 ± 0.13 |
| Jelly 2 | ND | 17.43 ± 23.88 | 82.57 ± 23.88 | 298.83 ± 82.98 | −32.57 ± 0.76 | 2.33 ± 0.37 |
| Sauce 1 | ND | 63.40 ± 34.26 | 36.60 ± 34.26 | 316.07 ± 35.30 | −25.20 ± 1.15 | 3.70 ± 0.41 |
| Sauce 2 | ND | 11.33 ± 17.76 | 88.67 ± 17.76 | 461.47 ± 6.76 | −36.80 ± 0.78 | 0.94 ± 0.04 |
| Snack | ND | 2.87 ± 4.13 | 97.13 ± 4.13 | 356.63 ± 8.45 | −25.93 ± 0.47 | 1.27 ± 0.08 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.-S.; Yu, J.; Kim, H.-M.; Oh, J.-M.; Choi, S.-J. Food Additive Titanium Dioxide and Its Fate in Commercial Foods. Nanomaterials 2019, 9, 1175. https://doi.org/10.3390/nano9081175
Hwang J-S, Yu J, Kim H-M, Oh J-M, Choi S-J. Food Additive Titanium Dioxide and Its Fate in Commercial Foods. Nanomaterials. 2019; 9(8):1175. https://doi.org/10.3390/nano9081175
Chicago/Turabian StyleHwang, Ji-Soo, Jin Yu, Hyoung-Mi Kim, Jae-Min Oh, and Soo-Jin Choi. 2019. "Food Additive Titanium Dioxide and Its Fate in Commercial Foods" Nanomaterials 9, no. 8: 1175. https://doi.org/10.3390/nano9081175
APA StyleHwang, J. -S., Yu, J., Kim, H. -M., Oh, J. -M., & Choi, S. -J. (2019). Food Additive Titanium Dioxide and Its Fate in Commercial Foods. Nanomaterials, 9(8), 1175. https://doi.org/10.3390/nano9081175