Hydrophilic Silver Nanoparticles Loaded into Niosomes: Physical–Chemical Characterization in View of Biological Applications
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
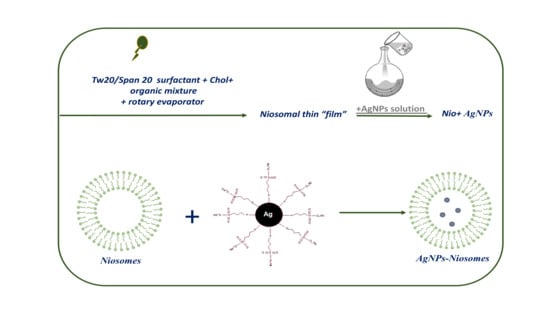
2.2. Preparation of AgNPs Loaded Niosomes
2.3. Characterizations
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clebak, K.T.; Malone, M.A. Skin Infections. Prim. Care 2018, 45, 433–454. [Google Scholar] [CrossRef]
- Manaresi, E.; Gallinella, G. Advances in the Development of Antiviral Strategies against Parvovirus B19. Viruses 2019, 11, 659. [Google Scholar] [CrossRef]
- Hutnick, M.A.; Pokorski, J.K. Polymeric Interventions for Microbial Infections: A Review. Mol. Pharm. 2018, 158, 2910–2921. [Google Scholar] [CrossRef]
- Friedman, D.Z.P.; Schwartz, I.S. Emerging Fungal Infections: New Patients, New Patterns, and New Pathogens. J. Fungi 2019, 5, 67. [Google Scholar] [CrossRef]
- Bunschoten, A.; Welling, M.M.; Termaat, M.F.; Sathekge, M.; van Leeuwen, F.W.B. Development and Prospects of Dedicated Tracers for the Molecular Imaging of Bacterial Infections. Bioconjug. Chem. 2013, 24, 1971–1989. [Google Scholar] [CrossRef]
- Nordøy, I.; Hesstvedt, L.; Torp Andersen, C.; Mylvaganam, H.; Kols, N.I.; Falch, B.M.; Tofteland, S.; Müller, F.; Denning, D.W. An Estimate of the Burden of Fungal Disease in Norway. J. Fungi 2018, 4, 29. [Google Scholar] [CrossRef]
- Wagner, S.; Sommer, R.; Hinsberger, S.; Lu, C.; Hartmann, R.W.; Empting, M.; Titz, A. Novel Strategies for the Treatment of Pseudomonas aeruginosa Infections. J. Med. Chem. 2016, 59, 5929–5969. [Google Scholar] [CrossRef]
- Frieria, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Das, S.; Singh, S.; Gajamer, V.R.; Pradhan, N.; Lepcha, Y.D.; Tiwari, H.K. Prevalence of antibiotic resistance in commensal Escherichia coli among the children in rural hill communities of Northeast India. PLoS ONE 2018, 13, e0199179. [Google Scholar] [CrossRef]
- Mu, H.; Tang, J.; Liu, Q.; Sun, C.; Wang, T.; Duan, J. Potent antibacterial nanoparticles against biofilm and intracellular bacteria. Sci. Rep. 2016, 6, 18877. [Google Scholar] [CrossRef]
- Venditti, I. Engineered gold-based nanomaterials: Morphologies and functionalities in biomedical applications. A mini review. Bioengineering 2019, 6, 53. [Google Scholar] [CrossRef]
- Porcaro, F.; Battocchio, C.; Antoccia, A.; Fratoddi, I.; Venditti, I.; Fracassi, A.; Luisetto, I.; Russo, M.; Polzonetti, G. Synthesis of functionalized gold nanoparticles capped with 3-mercapto-1-propansulfonate and 1-thioglucose mixed thiols and “in vitro” bioresponse. Colloids Surf. B Biointerfaces 2016, 142, 408–416. [Google Scholar] [CrossRef]
- Porcaro, F.; Carlini, L.; Ugolini, A.; Visaggio, D.; Visca, P.; Fratoddi, I.; Venditti, I.; Meneghini, C.; Simonelli, L.; Marini, C. Synthesis and structural characterization of silver nanoparticles stabilized with 3-mercapto-1-propansulfonate and 1-thioglucose mixed thiols for antibacterial applications. Materials 2016, 9, 1028. [Google Scholar] [CrossRef]
- Hong, W.; Zhao, Y.; Guo, Y.; Huang, C.; Qiu, P.; Zhu, J.; Chu, X.; Shi, H.; Liu, M. PEGylated Self-Assembled Nano-Bacitracin A: Probing the Antibacterial Mechanism and Real-Time Tracing of Target Delivery in Vivo. ACS Appl. Mater. Interfaces 2018, 10, 10688–10705. [Google Scholar] [CrossRef]
- Fratoddi, I.; Benassi, L.; Botti, E.; Vaschieri, C.; Venditti, I.; Bessar, H.; Samir, M.A.; Azzoni, P.; Magnoni, C.; Costanzo, A. Effects of topical methotrexate loaded gold nanoparticle in cutaneous inflammatory mouse model. Nanomedicine 2019, 17, 276–286. [Google Scholar] [CrossRef]
- Venditti, I.; Testa, G.; Sciubba, F.; Carlini, L.; Porcaro, F.; Meneghini, C.; Mobilio, S.; Battocchio, C.; Fratoddi, I. Hydrophilic metal nanoparticles functionalized by 2-Diethylaminoethanethiol: A close look at the metal–ligand interaction and interface chemical structure. J. Phys. Chem. C 2017, 121, 8002–8013. [Google Scholar] [CrossRef]
- Prosposito, P.; Mochi, F.; Ciotta, E.; Casalboni, M.; De Matteis, F.; Venditti, I.; Fontana, L.; Testa, G.; Fratoddi, I. Hydrophilic silver nanoparticles with tunable optical properties: Application for the detection of heavy metals in water. Beilstein J. Nanotechnol. 2016, 7, 1654–1661. [Google Scholar] [CrossRef]
- Mochi, F.; Burratti, L.; Fratoddi, I.; Venditti, I.; Battocchio, C.; Carlini, L.; Iucci, G.; Casalboni, M.; De Matteis, F.; Casciardi, S.; et al. Interaction of colloidal silver nanoparticles with Co2+ and Ni2+ in water for sensing application. Nanomaterials 2018, 8, 488. [Google Scholar] [CrossRef]
- Corsi, P.; Venditti, I.; Battocchio, C.; Meneghini, C.; Bruni, F.; Prosposito, P.; Mochi, F.; Capone, B. Designing an Optimal Ion Adsorber at the Nanoscale: The Unusual Nucleation of AgNP/Co2+–Ni2+ Binary Mixtures. J. Phys. Chem. C 2019, 123, 3855–3860. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yang, C.; Yin, X.; Sun, Y.; Weng, J.; Zhou, J.; Feng, B. Inflammatory responses to micro/nano-structured titanium surfaces with silver nanoparticles in vitro. J. Mater. Chem. B 2019, 7, 3546–3559. [Google Scholar] [CrossRef]
- Burdușel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Aazam, E.S.; Zaheer, Z. Growth of Ag-nanoparticles in an aqueous solution and their antimicrobial activities against Gram positive, Gram negative bacterial strains and Candida fungus. Bioprocess Biosyst. Eng. 2016, 39, 575–584. [Google Scholar] [CrossRef]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Techc, R.Y.M.; Kalaichelvan, P.T.; Venketesan, R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria. Nanomedicine 2010, 6, 103–109. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, e1701503. [Google Scholar] [CrossRef]
- Jinu, U.; Jayalakshmi, N.; Sujima Anbu, A.; Mahendran, D.; Sahi, S.; Venkatachalam, P. Biofabrication of Cubic Phase Silver Nanoparticles Loaded with Phytochemicals from Solanum nigrum Leaf Extracts for Potential Antibacterial, Antibiofilm and Antioxidant Activities Against MDR Human Pathogens. J. Clust. Sci. 2017, 28, 489–505. [Google Scholar] [CrossRef]
- Gopinath, P.M.; Narchonai, G.; Dhanasekaran, D.; Ranjani, A.; Thajuddin, N. Mycosynthesis, characterization and antibacterial properties of AgNPs against multidrug resistant (MDR) bacterial pathogens of female infertility cases. Asian J. Pharm. Sci. 2015, 10, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Sanyasi, S.; Majhi, R.K.; Kumar, S.; Mishra, M.; Ghosh, A.; Suar, M.; Satyam, P.V.; Mohapatra, H.; Goswami, C.; Goswami, L. Polysaccharide-capped silver Nanoparticles inhibit biofilm formation and eliminate multi-drug-resistant bacteria by disrupting bacterial cytoskeleton with reduced cytotoxicity towards mammalian cells. Sci. Rep. 2016, 6, 24929. [Google Scholar] [CrossRef]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine 2007, 3, 168–171. [Google Scholar] [CrossRef]
- Maneerung, T.; Tokura, S.; Rujiravanit, R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr. Polym. 2008, 72, 43–51. [Google Scholar] [CrossRef]
- Corrêa, J.M.; Mori, M.; Sanches, H.L.; Cruz, A.D.D.; Poiate, E.; Poiate, I.A.V.P. Silver nanoparticles in dental biomaterials. Int. J. Biomater. 2015, 2015, 485275. [Google Scholar] [CrossRef]
- Knetsch, M.L.; Koole, L.H. New strategies in the development of antimicrobial coatings: The example of increasing usage of silver and silver nanoparticles. Polymers 2011, 3, 340–366. [Google Scholar] [CrossRef]
- Samuel, U.; Guggenbichler, J. Prevention of catheter-related infections: The potential of a new nano-silver impregnated catheter. Int. J. Antimicrob. Agents 2004, 23, 75–78. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Ran, L.; Zou, Y.; Cheng, J.; Lu, F. Silver nanoparticles in situ synthesized by polysaccharides from Sanghuangporus sanghuang and composites with chitosan to prepare scaffolds for the regeneration of infected full-thickness skin defects. Int. J. Biol. Macromol. 2019, 125, 392–403. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef]
- Elbehiry, A.; Al-Dubaib, M.; Marzouk, E.; Moussa, I. Antibacterial effects and resistance induction of silver and gold nanoparticles against Staphylococcus aureus-induced mastitis and the potential toxicity in rats. MicrobiologyOpen 2019, 8, e698. [Google Scholar] [CrossRef]
- Das, A.; Debasis, S.; Jitendra, B.; Mahesh, S. Confinement induced formation of silver nanoparticles in self-assembled micro-granules. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 185–193. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, Y.-f.; Xu, J.; Liang, P.; Liu, Z.-g.; Wang, J.; Zhang, D.; Dong, Q.-m.; Shen, W.-m.; Zhuang, S.-l. Unveiling the growth mechanism of SiO2/Ag hybrid nanospheres and using for Surface Enhanced Raman Scattering detection. Appl. Surf. Sci. 2019, 463, 115–120. [Google Scholar] [CrossRef]
- Kumar, K.A.; John, J.; Sooraj, T.; Raj, S.A.; Unnikrishnan, N.; Selvaraj, N.B. Surface plasmon response of silver nanoparticles doped silica synthesised via sol-gel route. Appl. Surf. Sci. 2019, 472, 40–45. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, H.; Cui, H.; Xu, M.; Cao, S.; Zheng, G.; Dong, M. Preparation and antibacterial activities of hollow silica–Ag spheres. Colloids Surf. B Biointerfaces 2013, 101, 97–100. [Google Scholar] [CrossRef]
- Nishanthi, S.; Yadav, K.K.; Baruah, A.; Vaghasiya, K.; Verma, R.K.; Ganguli, A.K.; Jha, M. Nanostructured silver decorated hollow silica and their application in the treatment of microbial contaminated water at room temperature. New J. Chem. 2019, 43, 8993–9001. [Google Scholar] [CrossRef]
- Gao, A.; Chen, H.; Hou, A.; Xie, K. Efficient antimicrobial silk composites using synergistic effects of violacein and silver nanoparticles. Mater. Sci. Eng. C 2019, 103, 109821. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Su, L.; Luan, Y.; Du, X.; Zhang, X. Effect of surface topology morphologies of silica nanocarriers on the loading of Ag nanoparticles and antibacterial performance. J. Alloys Compd. 2019, 783, 136–144. [Google Scholar] [CrossRef]
- Marianecci, C.; Di Marzio, L.; Rinaldi, F.; Celia, C.; Paolino, D.; Alhaique, F.; Esposito, S.; Carafa, M. Niosomes from 80s to present: The state of the art. Adv. Colloid Interface Sci. 2014, 205, 187–206. [Google Scholar] [CrossRef]
- Venditti, I. Morphologies and functionalities of polymeric nanocarriers as chemical tools for drug delivery: A review. J. King Saud Univ. Sci. 2019, 31, 398–411. [Google Scholar] [CrossRef]
- Ge, X.; Wei, M.; He, S.; Yuan, W.-E. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef]
- Yusuf, A.; Brophy, A.; Gorey, B.; Casey, A. Liposomal encapsulation of silver nanoparticles enhances cytotoxicity and causes induction of reactive oxygen species-independent apoptosis. J. Appl. Toxicol. 2018, 38, 616–627. [Google Scholar] [CrossRef]
- Rinaldi, F.; Seguella, L.; Gigli, S.; Hanieh, P.; Del Favero, E.; Cantù, L.; Pesce, M.; Sarnelli, G.; Marianecci, C.; Esposito, G. In Pentasomes: An innovative nose-to-brain pentamidine delivery blunts MPTP parkinsonism in mice. J. Control. Release 2019, 294, 17–26. [Google Scholar] [CrossRef]
- Rinaldi, F.; Hanieh, P.N.; Chan, L.K.N.; Angeloni, L.; Passeri, D.; Rossi, M.; Wang, J.T.-W.; Imbriano, A.; Carafa, M.; Marianecci, C. Chitosan Glutamate-Coated Niosomes: A Proposal for Nose-to-Brain Delivery. Pharmaceutics 2018, 10, 38. [Google Scholar] [CrossRef]
- De Angelis, R.; Venditti, I.; Fratoddi, I.; De Matteis, F.; Prosposito, P.; Cacciotti, I.; D’Amico, L.; Nanni, F.; Yadav, A.; Casalboni, M.; et al. From nanospheres to microribbons: Self-assembled Eosin Y doped PMMA nanoparticles as photonic crystals. J. Colloid Interf. Sci. 2014, 414, 24–32. [Google Scholar] [CrossRef]
- Sennato, S.; Bordi, F.; Cametti, C.; Marianecci, C.; Carafa, M.; Cametti, M. Hybrid niosome complexation in the presence of oppositely charged polyions. J. Phys. Chem. B 2008, 112, 3720–3727. [Google Scholar] [CrossRef]
- Rao, H.S.P.; Desai, A.; Sarkar, I.; Mohapatra, M.; Mishra, A.K. Photophysical behavior of a new cholesterol attached coumarin derivative and fluorescence spectroscopic studies on its interaction with bile salt systems and lipid bilayer membranes. Phys. Chem. Chem. Phys. 2014, 16, 1247–1256. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Lentz, B.R. Membrane “fluidity” as detected by diphenylhexatriene probes. Chem. Phys. Lipids 1989, 50, 171–190. [Google Scholar] [CrossRef]
- Zachariasse, K.A. Intramolecular excimer formation with diarylalkanes as a microfluidity probe for sodium dodecyl sulphate micelles. Chem. Phys. Lett. 1978, 57, 429–432. [Google Scholar] [CrossRef]
- Ingallina, C.; Rinaldi, F.; Bogni, A.; Ponti, J.; Passeri, D.; Reggente, M.; Rossi, M.; Kinsner-Ovaskainen, A.; Mehn, D.; Rossi, F. Niosomal approach to brain delivery: Development, characterization and in vitro toxicological studies. Int. J. Pharm. 2016, 511, 969–982. [Google Scholar] [CrossRef]
- Rinaldi, F.; Hanieh, P.N.; Del Favero, E.; Rondelli, V.; Brocca, P.; Pereira, M.C.; Andreev, O.A.; Reshetnyak, Y.K.; Marianecci, C.; Carafa, M. Decoration of nanovesicles with pH (low) insertion peptide (pHLIP) for targeted delivery. Nanoscale Res. Lett. 2018, 13, 391. [Google Scholar] [CrossRef]
- Carafa, M.; Marianecci, C.; Rinaldi, F.; Santucci, E.; Tampucci, S.; Monti, D. Span® and Tween® neutral and pH-sensitive vesicles: Characterization and in vitro skin permeation. J. Liposome Res. 2009, 19, 332–340. [Google Scholar] [CrossRef]
- Marianecci, C.; Di Marzio, L.; Del Favero, E.; Cantù, L.; Brocca, P.; Rondelli, V.; Rinaldi, F.; Dini, L.; Serra, A.; Decuzzi, P. Niosomes as drug nanovectors: Multiscale pH-dependent structural response. Langmuir 2016, 32, 1241–1249. [Google Scholar] [CrossRef]
- Palchetti, S.; Colapicchioni, V.; Digiacomo, L.; Caracciolo, G.; Pozzi, D.; Capriotti, A.L.; La Barbera, G.; Laganà, A. The protein corona of circulating PEGylated liposomes. Biochim. Biophys. Acta 2016, 1858, 189–196. [Google Scholar] [CrossRef]







| Samples ID | Hydrodynamic Diameter (nm) ± SD | ζ-Potential (mV) ± SD | PDI ± SD |
|---|---|---|---|
| NioTw20 | 136.1 ± 2.0 | −32.8 ± 0.3 | 0.38 ± 0.01 |
| NioTw20 + AgNPs | 140.3 ± 3.9 | −33.1 ± 1.4 | 0.40 ± 0.01 |
| NioSp20 | 230.2 ± 5.9 | −42.7 ± 2.3 | 0.35 ± 0.01 |
| NioSp20 + AgNPs | 251.7 ± 6.0 | −42.9 ± 1.2 | 0.40 ± 0.01 |
| Samples ID | Entrapment Efficiency (%) |
|---|---|
| NioTw20 + AgNPs | <1 |
| NioSp20 + AgNPs | 4 |
| Samples ID | Fluidity (Anisotropy) | Microviscosity (IE/I3) | Polarity (I1/I3) |
|---|---|---|---|
| NioTw20 | 0.10 | 0.90 | 0.90 |
| NioTw20 + AgNPs | 0.11 | 0.90 | 0.90 |
| NioSp20 | 0.10 | 1.01 | 0.94 |
| NioSp20 + AgNPs | 0.11 | 1.03 | 0.90 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi, F.; del Favero, E.; Moeller, J.; Hanieh, P.N.; Passeri, D.; Rossi, M.; Angeloni, L.; Venditti, I.; Marianecci, C.; Carafa, M.; et al. Hydrophilic Silver Nanoparticles Loaded into Niosomes: Physical–Chemical Characterization in View of Biological Applications. Nanomaterials 2019, 9, 1177. https://doi.org/10.3390/nano9081177
Rinaldi F, del Favero E, Moeller J, Hanieh PN, Passeri D, Rossi M, Angeloni L, Venditti I, Marianecci C, Carafa M, et al. Hydrophilic Silver Nanoparticles Loaded into Niosomes: Physical–Chemical Characterization in View of Biological Applications. Nanomaterials. 2019; 9(8):1177. https://doi.org/10.3390/nano9081177
Chicago/Turabian StyleRinaldi, Federica, Elena del Favero, Johannes Moeller, Patrizia Nadia Hanieh, Daniele Passeri, Marco Rossi, Livia Angeloni, Iole Venditti, Carlotta Marianecci, Maria Carafa, and et al. 2019. "Hydrophilic Silver Nanoparticles Loaded into Niosomes: Physical–Chemical Characterization in View of Biological Applications" Nanomaterials 9, no. 8: 1177. https://doi.org/10.3390/nano9081177
APA StyleRinaldi, F., del Favero, E., Moeller, J., Hanieh, P. N., Passeri, D., Rossi, M., Angeloni, L., Venditti, I., Marianecci, C., Carafa, M., & Fratoddi, I. (2019). Hydrophilic Silver Nanoparticles Loaded into Niosomes: Physical–Chemical Characterization in View of Biological Applications. Nanomaterials, 9(8), 1177. https://doi.org/10.3390/nano9081177