The Role of Fluorinated IL as an Interfacial Agent in P(VDF-CTFE)/Graphene Composite Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GO and rGO
2.3. Modification of GO and rGO with IL: GO-IL and rGO-IL
2.4. Preparation of P(VDF-CTFE)/GO, P(VDF-CTFE)/GO-IL, and P(VDF-CTFE)/rGO-IL Composite Films
2.5. Characterization
3. Results
3.1. Characterizations of the Fillers
3.1.1. Preparation of GO, rGO, GO-IL, and rGO-IL
3.1.2. New Interaction Model: Hydrogen Bond C–F∙∙∙H–N between rGO and IL
3.2. Characterizations of the Composites
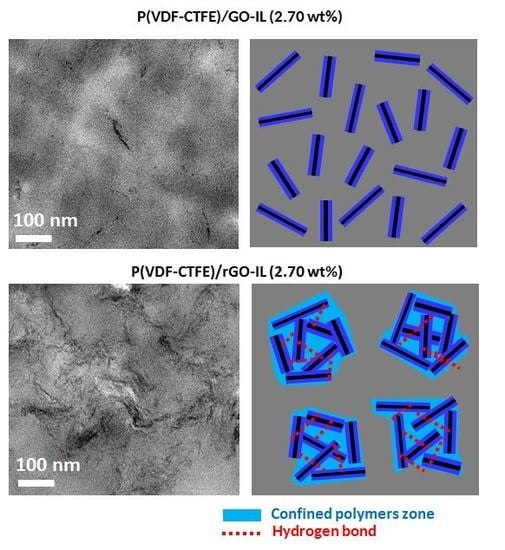
3.2.1. Dispersion Morphology
3.2.2. Crystalline Phase: Total γ-Phase Transformation
3.2.3. Crystallization Behavior: Heterogeneous Nucleation Effect
3.2.4. Relaxation Behavior
3.2.5. Electrical Conductivity of P(VDF-CTFE)/GO Composites
3.2.6. Dielectric Properties of P(VDF-CTFE)/IL-Modified Graphene Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, J.; Wang, C.; Shen, T.; Song, H.; Li, D.; Zhao, R.; Wang, X. Engineering the Dimensional Interface of BiVO4-2D Reduced Graphene Oxide (RGO) Nanocomposite for Enhanced Visible Light Photocatalytic Performance. Nanomaterials 2019, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/polymer nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Hu, K.; Kulkarni, D.D.; Choi, I.; Tsukruk, V.V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Johnson, D.W.; Dobson, B.P.; Coleman, K.S. A manufacturing perspective on graphene dispersions. Curr. Opin. Colloid Interface Sci. 2015, 20, 367–382. [Google Scholar] [CrossRef] [Green Version]
- Layek, R.K.; Nandi, A.K. A review on synthesis and properties of polymer functionalized graphene. Polymer 2013, 54, 5087–5103. [Google Scholar] [CrossRef] [Green Version]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Jung, I.; Dikin, D.A.; Piner, R.D.; Ruoff, R.S. Tunable electrical conductivity of individual graphene oxide sheets reduced at “low” temperatures. Nano Lett. 2008, 8, 4283–4287. [Google Scholar] [CrossRef]
- Layek, R.K.; Samanta, S.; Chatterjee, D.P.; Nandi, A.K. Physical and mechanical properties of poly (methyl methacrylate)-functionalized graphene/poly (vinylidine fluoride) nanocomposites: Piezoelectric β polymorph formation. Polymer 2010, 51, 5846–5856. [Google Scholar] [CrossRef]
- Wang, D.; Bao, Y.; Zha, J.W.; Zhao, J.; Dang, Z.M.; Hu, G.H. Improved dielectric properties of nanocomposites based on poly (vinylidene fluoride) and poly (vinyl alcohol)-functionalized graphene. ACS Appl. Mater. Interfaces 2012, 4, 6273–6279. [Google Scholar] [CrossRef]
- Han, K.; Li, Q.; Chen, Z.; Gadinski, M.R.; Dong, L.; Xiong, C.; Wang, Q. Suppression of energy dissipation and enhancement of breakdown strength in ferroelectric polymer–graphene percolative composites. J. Mater. Chem. C 2013, 1, 7034–7042. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.Z.; Zhu, L.; Zhong, G.J.; Li, Z.M. Role of ion–dipole interactions in nucleation of gamma poly (vinylidene fluoride) in the presence of graphene oxide during melt crystallization. J. Phys. Chem. B 2012, 116, 14951–14960. [Google Scholar] [CrossRef]
- Wang, J.; Wu, J.; Xu, W.; Zhang, Q.; Fu, Q. Preparation of poly (vinylidene fluoride) films with excellent electric property, improved dielectric property and dominant polar crystalline forms by adding a quaternary phosphorus salt functionalized graphene. Compos. Sci. Technol. 2014, 91, 1–7. [Google Scholar] [CrossRef]
- Maity, N.; Mandal, A.; Nandi, A.K. Interface engineering of ionic liquid integrated graphene in poly (vinylidene fluoride) matrix yielding magnificent improvement in mechanical, electrical and dielectric properties. Polymer 2015, 65, 154–167. [Google Scholar] [CrossRef]
- Livi, S.; Pham, T.N.; Gérard, J.F.; Duchet-Rumeau, J. Supercritical CO2–ionic liquids: Green combination for preparing foams. Chem. Eng. J. 2014, 240, 534–540. [Google Scholar] [CrossRef]
- Livi, S.; Duchet-Rumeau, J.; Gérard, J.F. Tailoring of interfacial properties by ionic liquids in a fluorinated matrix based nanocomposites. Eur. Polym. J. 2011, 47, 1361–1369. [Google Scholar] [CrossRef]
- Yang, Y.K.; He, C.E.; Peng, R.G.; Baji, A.; Du, X.S.; Huang, Y.L.; Xie, X.L.; Mai, Y.W. Non-covalently modified graphene sheets by imidazolium ionic liquids for multifunctional polymer nanocomposites. J. Mater. Chem. 2012, 22, 5666–5675. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Martinez-Alonso, A.; Tascon, J.M.D. Graphene oxide dispersions in organic solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef]
- Jeong, H.K.; Lee, Y.P.; Jin, M.H.; Kim, E.S.; Bae, J.J.; Lee, Y.H. Thermal stability of graphite oxide. Chem. Phys. Lett. 2009, 470, 255–258. [Google Scholar] [CrossRef]
- Jeong, H.K.; Lee, Y.P.; Lahaye, R.J.W.E.; Park, M.H.; An, K.H.; Kim, I.J.; Yang, C.W.; Park, C.Y.; Ruoff, R.S.; Lee, Y.H. Evidence of graphitic AB stacking order of graphite oxides. J. Am. Chem. Soc. 2008, 130, 1362–1366. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Abidi, N.; Cabrales, L. Wettability and surface free energy of graphene films. Langmuir 2009, 25, 11078–11081. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Wang, S. Enhancing thermoelectric properties of organic composites through hierarchical nanostructures. Sci. Rep. 2013, 3, 3448. [Google Scholar] [CrossRef] [Green Version]
- Kwizera, P.; Dresselhaus, M.S.; Uhlmann, D.R. The microstructure of intercalated graphite fibers. Carbon 1982, 20, 387–394. [Google Scholar] [CrossRef]
- Zheng, X.; Yu, H.; Yue, S.; Xing, R.; Zhang, Q.; Liu, Y.; Zhang, B. Functionalization of graphene and dielectric property relationships in PVDF/graphene nanosheets composites. Int. J. Electrochem. Sci. 2018, 13, 1–13. [Google Scholar] [CrossRef]
- Khanra, P.; Lee, C.N.; Kuila, T.; Kim, N.H.; Park, M.J.; Lee, J.H. 7, 7, 8, 8-Tetracyanoquinodimethane-assisted one-step electrochemical exfoliation of graphite and its performance as an electrode material. Nanoscale 2014, 6, 4864–4873. [Google Scholar] [CrossRef]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and thermal reduction of graphene oxide: Reaction mechanisms, product structures, and reaction design. J. Phys. Chem. C 2009, 114, 832–842. [Google Scholar] [CrossRef]
- Tong, W.; Zhang, Y.; Yu, L.; Luan, X.; An, Q.; Zhang, Q.; Lv, F.; Chu, P.K.; Shen, B.; Zhang, Z. Novel Method for the Fabrication of Flexible Film with Oriented Arrays of Graphene in Poly (vinylidene fluoride-co-hexafluoropropylene) with Low Dielectric Loss. J. Phys. Chem. C 2014, 118, 10567–10573. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Xu, C.; Sun, J.; Gao, L. Facile one-step hydrazine-assisted solvothermal synthesis of nitrogen-doped reduced graphene oxide: Reduction effect and mechanisms. RSC Adv. 2013, 3, 1194–1200. [Google Scholar] [CrossRef]
- Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar]
- Almadhoun, M.N.; Hedhili, M.N.; Odeh, I.N.; Xavier, P.; Bhansali, U.S.; Alshareef, H.N. Influence of stacking morphology and edge nitrogen doping on the dielectric performance of graphene–polymer nanocomposites. Chem. Mater. 2014, 26, 2856–2861. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cancado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.J. The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2, 032183. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Puech, N.; Zakri, C.; Grelet, E.; Moulton, S.E.; Wallace, G.G.; Gambhir, S.; Blanc, C.; Anglaret, E.; Poulin, P. Liquid crystallinity and dimensions of surfactant-stabilized sheets of reduced graphene oxide. J. Phys. Chem. Lett. 2012, 3, 2425–2430. [Google Scholar] [CrossRef]
- Yuan, J.; Luna, A.; Neri, W.; Zakri, C.; Schilling, T.; Colin, A.; Poulin, P. Graphene liquid crystal retarded percolation for new high-k materials. Nat. Commun. 2015, 6, 8700. [Google Scholar] [CrossRef]
- Balapanuru, J.; Yang, J.X.; Xiao, S.; Bao, Q.; Jahan, M.; Polavarapu, L.; Wei, J.; Xu, Q.H.; Loh, K.P. A graphene oxide–organic dye ionic complex with DNA-sensing and optical-limiting properties. Angew. Chem. Int. Ed. 2010, 49, 6549–6553. [Google Scholar] [CrossRef]
- Chaudhari, S.R.; Mogurampelly, S.; Suryaprakash, N. Engagement of CF3 Group in N–H···F–C Hydrogen Bond in the Solution State: NMR Spectroscopy and MD Simulation Studies. J. Phys. Chem. B 2013, 117, 1123–1129. [Google Scholar] [CrossRef]
- Gupta, M.; Gleason, K.K. Initiated chemical vapor deposition of poly (1H,1H,2H,2H-perfluorodecyl acrylate) thin films. Langmuir 2006, 22, 10047–10052. [Google Scholar] [CrossRef]
- Lopes, A.C.; Costa, C.M.; Tavares, C.J.; Neves, I.C.; Lanceros-Mendez, S. Nucleation of the electroactive γ phase and enhancement of the optical transparency in low filler content poly(vinylidene)/clay nanocomposites. J. Phys. Chem. C 2011, 115, 18076–18082. [Google Scholar] [CrossRef]
- Barrau, S.; Ferri, A.; Da Costa, A.; Defebvin, J.; Leroy, S.; Desfeux, R.; Lefebvre, J.-M. Nanoscale investigations of α- and γ–crystal phases in PVDF-based nanocomposites. ACS Appl. Mater. Interfaces 2018, 10, 13092–13099. [Google Scholar] [CrossRef]
- Li, W.; Meng, Q.; Zheng, Y.; Zhang, Z.; Xia, W.; Xu, Z. Electric energy storage properties of poly (vinylidene fluoride). Appl. Phys. Lett. 2010, 96, 192905. [Google Scholar]
- Xing, C.; Zhao, L.; You, J.; Dong, W.; Cao, X.; Li, Y. Impact of ionic liquid-modified multiwalled carbon nanotubes on the crystallization behavior of poly (vinylidene fluoride). J. Phys. Chem. B 2012, 116, 8312–8320. [Google Scholar] [CrossRef]
- Mandal, A.; Nandi, A.K. Ionic liquid integrated multiwalled carbon nanotube in a poly (vinylidene fluoride) matrix: Formation of a piezoelectric β-polymorph with significant reinforcement and conductivity improvement. ACS Appl. Mater. Interfaces 2013, 5, 747–760. [Google Scholar] [CrossRef]
- Sousa, R.E.; Ferreira, J.C.C.; Costa, C.M.; Machado, A.V.; Silva, M.M.; Lanceros-Mendez, S. Tailoring poly (vinylidene fluoride-co-chlorotrifluoroethylene) microstructure and physicochemical properties by exploring its binary phase diagram with dimethylformamide. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 761–773. [Google Scholar] [CrossRef]
- Yang, J.; Pruvost, S.; Livi, S.; Duchet-Rumeau, J. Understanding of versatile and tunable nanostructuration of ionic liquids on fluorinated copolymer. Macromolecules 2015, 48, 4581–4590. [Google Scholar] [CrossRef]
- Xu, J.Z.; Zhong, G.J.; Hsiao, B.S.; Fu, Q.; Li, Z.M. Low-dimensional carbonaceous nanofiller induced polymer crystallization. Prog. Polym. Sci. 2014, 39, 555–593. [Google Scholar] [CrossRef]
- Mijovic, J.; Sy, J.W.; Kwei, T.K. Reorientational dynamics of dipoles in poly (vinylidene fluoride)/poly (methyl methacrylate)(PVDF/PMMA) blends by dielectric spectroscopy. Macromolecules 1997, 30, 3042–3050. [Google Scholar] [CrossRef]
- Mack, J.J.; Viculis, L.M.; Ali, A.; Luoh, R.; Yang, G.; Hahn, H.T.; Ko, F.K.; Kaner, R.B. Graphite nanoplatelet reinforcement of electrospun polyacrylonitrile nanofibers. Adv. Mater. 2005, 17, 77–80. [Google Scholar] [CrossRef]
- Chen, G.; Weng, W.; Wu, D.; Wu, C.; Lu, J.; Wang, P.; Chen, X. Preparation and characterization of graphite nanosheets from ultrasonic powdering technique. Carbon 2004, 42, 753–759. [Google Scholar] [CrossRef]
- Zheng, G.; Wu, J.; Wang, W.; Pan, C. Characterizations of expanded graphite/polymer composites prepared by in situ polymerization. Carbon 2004, 42, 2839–2847. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Podgórski, A. Photochemical and thermal behaviours of poly (vinyl alcohol)/graphite oxide composites. Polym. Degrad. Stab. 2007, 92, 939–946. [Google Scholar] [CrossRef]
- Yin, X.; Capsal, J.F.; Guyomar, D. A comprehensive investigation of poly (vinylidene fluoride-trifluoroethylene-chlorofluoroethylene) terpolymer nanocomposites with carbon black for electrostrictive applications. Appl. Phys. Lett. 2014, 104, 052913. [Google Scholar] [CrossRef]


















| Sample | Filler Content (wt%) | ||||||
|---|---|---|---|---|---|---|---|
| P(VDF-CTFE)/IL | 0.5 | 1 | 2 | – | – | – | – |
| P(VDF-CTFE)/GO | 0.27 | 0.55 | 0.81 | – | – | – | – |
| P(VDF-CTFE)/GO-IL | 0.27 | 0.55 | 0.81 | 1.08 | 1.36 | 2.16 | 2.70 |
| P(VDF-CTFE)/rGO-IL | 0.55 | 0.81 | 1.36 | 1.62 | 1.89 | 2.16 | 2.70 |
| Sample | 2θ (°) | d (Å) | β (°) | t (Å) | n |
|---|---|---|---|---|---|
| graphite | 26.5 | 3.4 | 0.15 | 538.3 | 158.3 |
| GO | 9.8 | 9.0 | 2.19 | 36.0 | 4.0 |
| rGO | 24.5 | 3.6 | 5.70 | 14.1 | 3.9 |
| GO-IL | 17.8 | 5.0 | 5.63 | 14.1 | 2.8 |
| Sample | –OH/C=C | C=O/C=C |
|---|---|---|
| GO | 3.9 | 2.2 |
| rGO | 1.8 | 0.2 |
| Sample | D Band (cm−1) | G Band (cm−1) | ID/IG | 2D Band (cm−1) |
|---|---|---|---|---|
| graphite | 1342 | 1571 | 0.069 | 2700 |
| GO | 1344 | 1582 | 1.056 | – |
| rGO | 1343 | 1576 | 1.110 | 2440, 2700 |
| GO-IL | 1344 | 1582 | 1.003 | – |
| rGO-IL | 1342 | 1578 | 1.113 | – |
| wt% | 0 | 0.55% | 0.81% | 1.36% | 1.62% | 1.89% | 2.16% | 2.70% |
|---|---|---|---|---|---|---|---|---|
| εr’ | 11.0 | 11.8 | 12.5 | 13.0 | 14.2 | 15.4 | 16.1 | 20.3 |
| tan δ | 0.030 | 0.030 | 0.024 | 0.026 | 0.025 | 0.032 | 0.026 | 0.032 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Pruvost, S.; Livi, S.; Duchet-Rumeau, J. The Role of Fluorinated IL as an Interfacial Agent in P(VDF-CTFE)/Graphene Composite Films. Nanomaterials 2019, 9, 1181. https://doi.org/10.3390/nano9081181
Yang J, Pruvost S, Livi S, Duchet-Rumeau J. The Role of Fluorinated IL as an Interfacial Agent in P(VDF-CTFE)/Graphene Composite Films. Nanomaterials. 2019; 9(8):1181. https://doi.org/10.3390/nano9081181
Chicago/Turabian StyleYang, Jing, Sébastien Pruvost, Sébastien Livi, and Jannick Duchet-Rumeau. 2019. "The Role of Fluorinated IL as an Interfacial Agent in P(VDF-CTFE)/Graphene Composite Films" Nanomaterials 9, no. 8: 1181. https://doi.org/10.3390/nano9081181
APA StyleYang, J., Pruvost, S., Livi, S., & Duchet-Rumeau, J. (2019). The Role of Fluorinated IL as an Interfacial Agent in P(VDF-CTFE)/Graphene Composite Films. Nanomaterials, 9(8), 1181. https://doi.org/10.3390/nano9081181