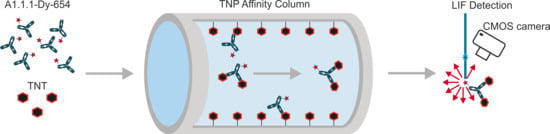
Fast Detection of 2,4,6-Trinitrotoluene (TNT) at ppt Level by a Laser-Induced Immunofluorometric Biosensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Buffers, Materials, and Equipment
2.2. Trinitrophenyl-BSA Conjugates and Indirect Competitive Enzyme-Linked Immunosorbent Assay (ELISA)
2.3. Manufacturing of Affinity Columns
2.4. Design and Synthesis of the A1.1.1 Fluorophore Conjugate
2.5. Fluorescence Detector
2.6. Measurements
3. Results
3.1. Fluorescence Detector
3.2. Semi-Automated Data Evaluation with Python
3.3. Antibody Selection, Validation, Labeling, and Determination of the Test Midpoint for 2,4,6-Trinitrotoluene (TNT)
3.4. 2,4,6-Trinitrophenyl-(TNP)-BSA Affinity Columns
3.5. Setup Optimization and Limit of Detection (LOD) of the Label
3.6. Performance of Affinity Column
3.7. TNT Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moore, D.S. Instrumentation for trace detection of high explosives. Rev. Sci. Instrum. 2004, 75, 2499–2512. [Google Scholar] [CrossRef]
- Caygill, J.S.; Davis, F.; Higson, S.P.J. Current trends in explosive detection techniques. Talanta 2012, 88, 14–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, K.E.; Greenfield, M.T.; McGrane, S.D.; Moore, D.S. Advances in explosives analysis-part I: Animal, chemical, ion, and mechanical methods. Anal. Bioanal. Chem. 2016, 408, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Onodera, T.; Toko, K. Towards an Electronic Dog Nose: Surface Plasmon Resonance Immunosensor for Security and Safety. Sensors (Basel) 2014, 14, 16586–16616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewing, R.G.; Atkinson, D.A.; Eiceman, G.A.; Ewing, G.J. A critical review of ion mobility spectrometry for the detection of explosives and explosive related compounds. Talanta 2001, 54, 515–529. [Google Scholar] [CrossRef]
- Crawford, C.L.; Hill, H.H. Evaluation of false positive responses by mass spectrometry and ion mobility spectrometry for the detection of trace explosives in complex samples. Anal. Chim. Acta 2013, 795, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Salinas, Y.; Martinez-Manez, R.; Marcos, M.D.; Sancenon, F.; Costero, A.M.; Parra, M.; Gil, S. Optical chemosensors and reagents to detect explosives. Chem. Soc. Rev. 2012, 41, 1261–1296. [Google Scholar] [CrossRef]
- Lubczyk, D.; Siering, C.; Lorgen, J.; Shifrina, Z.B.; Mullen, M.; Waldvogel, S.R. Simple and sensitive online detection of triacetone triperoxide explosive. Sens. Actuat. B-Chem. 2010, 143, 561–566. [Google Scholar] [CrossRef]
- Lubczyk, D.; Grill, M.; Baumgarten, M.; Waldvogel, S.R.; Mullen, K. Scaffold-Optimized Dendrimers for the Detection of the Triacetone Triperoxide Explosive Using Quartz Crystal Microbalances. Chempluschem 2012, 77, 102–105. [Google Scholar] [CrossRef]
- Kartha, K.K.; Babu, S.S.; Srinivasan, S.; Ajayaghosh, A. Attogram Sensing of Trinitrotoluene with a Self-Assembled Molecular Gelator. J. Am. Chem. Soc. 2012, 134, 4834–4841. [Google Scholar] [CrossRef]
- Yang, J.S.; Swager, T.M. Porous shape persistent fluorescent polymer films: An approach to TNT sensory materials. J. Am. Chem. Soc. 1998, 120, 5321–5322. [Google Scholar] [CrossRef]
- Chang, C.P.; Chao, C.Y.; Huang, J.H.; Li, A.K.; Hsu, C.S.; Lin, M.S.; Hsieh, B.R.; Su, A.C. Fluorescent conjugated polymer films as TNT chemosensors. Synth. Met. 2004, 144, 297–301. [Google Scholar] [CrossRef]
- Tu, R.Y.; Liu, B.H.; Wang, Z.Y.; Gao, D.M.; Wang, F.; Fang, Q.L.; Zhang, Z.P. Amine-capped ZnS-Mn2+ nanocrystals for fluorescence detection of trace TNT explosive. Anal. Chem. 2008, 80, 3458–3465. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.S.; Hu, Y.W.; Wang, X.; Zhang, L.L.; Li, F.H.; Han, D.X.; Li, Z.G.; Zhang, Q.X.; Wang, Z.X.; Niu, L. Fluorescence resonance energy transfer quenching at the surface of graphene quantum dots for ultrasensitive detection of TNT. Talanta 2012, 101, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, V.; Pramanik, S.; Kumar, M. Cyanide modulated fluorescent supramolecular assembly of a hexaphenylbenzene derivative for detection of trinitrotoluene at the attogram level. Chem. Commun. 2013, 49, 895–897. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, J.H.; Su, D.Y.; Xia, Q.D.; Chai, F.; Wang, C.G.; Qu, F.Y. Fluorescent detection of TNT and 4-nitrophenol by BSA Au nanoclusters. Dalton Trans. 2014, 43, 10057–10063. [Google Scholar] [CrossRef]
- Qian, J.J.; Qiu, L.G.; Wang, Y.M.; Yuan, Y.P.; Xie, A.J.; Shen, Y.H. Fabrication of magnetically separable fluorescent terbium-based MOF nanospheres for highly selective trace-level detection of TNT. Dalton Trans. 2014, 43, 3978–3983. [Google Scholar] [CrossRef]
- Mandal, M.; Balamurugan, R. Efficient Sensing of Trinitrotoluene Using a Photoluminescent Benzo[a]fluorenone Derivative. Chemistryselect 2019, 4, 10164–10168. [Google Scholar] [CrossRef]
- Meaney, M.S.; McGuffin, V.L. Luminescence-based methods for sensing and detection of explosives. Anal. Bioanal. Chem. 2008, 391, 2557–2576. [Google Scholar] [CrossRef]
- Smith, R.G.; D’Souza, N.; Nicklin, S. A review of biosensors and biologically-inspired systems for explosives detection. Analyst 2008, 133, 571–584. [Google Scholar] [CrossRef]
- Komikawa, T.; Tanaka, M.; Yanai, K.; Johnson, B.R.G.; Critchley, K.; Onodera, T.; Evans, S.D.; Toko, K.; Okochi, M. A bioinspired peptide matrix for the detection of 2,4,6-trinitrotoluene (TNT). Biosens. Bioelectron. 2020, 153, 112030. [Google Scholar] [CrossRef] [PubMed]
- Zarejousheghani, M.; Lorenz, W.; Vanninen, P.; Alizadeh, T.; Cammerer, M.; Borsdorf, H. Molecularly Imprinted Polymer Materials as Selective Recognition Sorbents for Explosives: A Review. Polymers 2019, 11, 888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.Z.; Florea, A.; Cristea, C.; Bessueille, F.; Vocanson, F.; Goutaland, F.; Zhang, A.D.; Sandulescu, R.; Lagarde, F.; Jaffrezic-Renault, N. 1,3,5-Trinitrotoluene detection by a molecularly imprinted polymer sensor based on electropolymerization of a microporous-metal-organic framework. Sens. Actuat. B-Chem. 2015, 207, 960–966. [Google Scholar] [CrossRef]
- Trammell, S.A.; Melde, B.J.; Zabetakis, D.; Deschamps, J.R.; Dinderman, M.A.; Johnson, B.J.; Kusterbeck, A.W. Electrochemical detection of TNT with in-line pre-concentration using imprinted diethylbenzene-bridged periodic mesoporous organosilicas. Sens. Actuat. B-Chem. 2011, 155, 737–744. [Google Scholar] [CrossRef]
- Stringer, R.C.; Gangopadhyay, S.; Grant, S.A. Detection of Nitroaromatic Explosives Using a Fluorescent-Labeled Imprinted Polymer. Anal. Chem. 2010, 82, 4015–4019. [Google Scholar] [CrossRef]
- Goldman, E.R.; Anderson, G.P.; Lebedev, N.; Lingerfelt, B.M.; Winter, P.T.; Patterson, C.H.; Mauro, J.M. Analysis of aqueous 2,4,6-trinitrotoluene (TNT) using a fluorescent displacement immunoassay. Anal. Bioanal. Chem. 2003, 375, 471–475. [Google Scholar] [CrossRef]
- Charles, P.T.; Adams, A.A.; Howell, P.B.; Trammell, S.A.; Deschamps, J.R.; Kusterbeck, A.W. Fluorescence-based Sensing of 2,4,6-Trinitrotoluene (TNT) Using a Multi-channeled Poly(methyl methacrylate) (PMMA) Microimmunosensor. Sensors (Basel) 2010, 10, 876–889. [Google Scholar] [CrossRef]
- Charles, P.T.; Rangasammy, J.G.; Anderson, G.P.; Romanoski, T.C.; Kusterbeck, A.W. Microcapillary reversed-displacement immunosensor for trace level detection of TNT in seawater. Anal. Chim. Acta 2004, 525, 199–204. [Google Scholar] [CrossRef]
- Shankaran, D.R.; Gobi, K.V.; Sakai, T.; Matsumoto, K.; Toko, K.; Miura, N. Surface plasmon resonance immunosensor for highly sensitive detection of 2,4,6-trinitrotoluene. Biosens. Bioelectron. 2005, 20, 1750–1756. [Google Scholar] [CrossRef]
- Onodera, T.; Mizuta, Y.; Horikawa, K.; Singh, P.; Matsumoto, K.; Miura, N.; Toko, K. Displacement Immunosensor Based on Surface Plasmon Resonance for Rapid and Highly Sensitive Detection of 2,4,6-Trinitrotoluene. Sens. Mater. 2011, 23, 39–52. [Google Scholar]
- Green, T.M.; Charles, P.T.; Anderson, G.P. Detection of 2,4,6-trinitrotoluene in seawater using a reversed-displacement immunosensor. Anal. Biochem. 2002, 310, 36–41. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Shankaran, D.R.; Kim, S.J.; Matsumoto, K.; Toko, K.; Miura, N. Surface plasmon resonance immunosensor using Au nanoparticle for detection of TNT. Sens. Actuat. B-Chem. 2008, 133, 467–472. [Google Scholar] [CrossRef]
- Whelan, J.P.; Kusterbeck, A.W.; Wemhoff, G.A.; Bredehorst, R.; Ligler, F.S. Continuous-Flow Immunosensor for Detection of Explosives. Anal. Chem. 1993, 65, 3561–3565. [Google Scholar] [CrossRef]
- Goldman, E.R.; Anderson, G.P.; Tran, P.T.; Mattoussi, H.; Charles, P.T.; Mauro, J.M. Conjugation of luminescent quantum dots with antibodies using an engineered adaptor protein to provide new reagents for fluoroimmunoassays. Anal. Chem. 2002, 74, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Bart, J.C.; Judd, L.L.; Kusterbeck, A.W. Environmental immunoassay for the explosive RDX using a fluorescent dye-labeled antigen and the continuous-flow immunosensor. Sens. Actuat. B-Chem. 1997, 39, 411–418. [Google Scholar] [CrossRef]
- Rabbany, S.Y.; Lane, W.J.; Marganski, W.A.; Kusterbeck, A.W.; Ligler, F.S. Trace detection of explosives using a membrane-based displacement immunoassay. J. Immunol. Methods 2000, 246, 69–77. [Google Scholar] [CrossRef]
- Mitchell, J. Small Molecule Immunosensing Using Surface Plasmon Resonance. Sensors 2010, 10, 7323–7346. [Google Scholar] [CrossRef] [Green Version]
- Onodera, T.; Ishii, R.; Yatabe, R.; Toko, K. Development of Sensor Surfaces Using Poly-(N-vinylformamide) for Sensitive Detection of 2,4,6-Trinitrotoluene by Displacement Method on a Surface Plasmon Resonance Sensor. Sens. Mater. 2016, 28, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Climent, E.; Groninger, D.; Hecht, M.; Walter, M.A.; Martinez-Manez, R.; Weller, M.G.; Sancenon, F.; Amoros, P.; Rurack, K. Selective, Sensitive, and Rapid Analysis with Lateral-Flow Assays Based on Antibody-Gated Dye-Delivery Systems: The Example of Triacetone Triperoxide. Chem.-Eur. J. 2013, 19, 4117–4122. [Google Scholar] [CrossRef]
- Ho, M.Y.; D’Souza, N.; Migliorato, P. Electrochemical Aptamer-Based Sandwich Assays for the Detection of Explosives. Anal. Chem. 2012, 84, 4245–4247. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, B.Y.; Jaworski, J.; Yokoyama, K.; Chung, W.J.; Wang, E.; Hong, S.; Majumdar, A.; Lee, S.W. Selective and Sensitive TNT Sensors Using Biomimetic Polydiacetylene-Coated CNT-FETs. Acs Nano 2011, 5, 2824–2830. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Zabetakis, D.; Acevedo-Velez, G.; Goldman, E.R.; Anderson, G.P. Comparison of an antibody and its recombinant derivative for the detection of the small molecule explosive 2,4,6-trinitrotoluene. Anal. Chim. Acta 2013, 759, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Kramer, P.M.; Kremmer, E.; Weber, C.M.; Ciumasu, I.M.; Forster, S.; Kettrup, A.A. Development of new rat monoclonal antibodies with different selectivities and sensitivities for 2,4,6-trinitrotoluene (TNT) and other nitroaromatic compounds. Anal. Bioanal. Chem. 2005, 382, 1919–1933. [Google Scholar] [CrossRef] [PubMed]
- Charles, P.T.; Shriver-Lake, L.C.; Francesconi, S.C.; Churilla, A.M.; Rangasammy, J.G.; Patterson, C.H.; Deschamps, J.R.; Kusterbeck, A.W. Characterization and performance evaluation of in vivo and in vitro produced monoclonal anti-TNT antibodies for the detection of TNT. J. Immunol. Methods 2004, 284, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Zeck, A.; Weller, M.G.; Niessner, R. Characterization of a monoclonal TNT-antibody by measurement of the cross-reactivities of nitroaromatic compounds. Fresen. J. Anal. Chem. 1999, 364, 113–120. [Google Scholar] [CrossRef]
- Julicher, P.; Mussenbrock, E.; Renneberg, R.; Cammann, K. Broadening the Antibody Specificity by Hapten Design for an Enzyme-Linked Immunoassay as an Improved Screening Method for the Determination of Nitroaromatic Residues in Soils. Anal. Chim. Acta 1995, 315, 279–287. [Google Scholar] [CrossRef]
- Hesse, A.; Biyikal, M.; Rurack, K.; Weller, M.G. Development of highly sensitive and selective antibodies for the detection of the explosive pentaerythritol tetranitrate (PETN) by bioisosteric replacement. J. Mol. Recognit. 2016, 29, 88–94. [Google Scholar] [CrossRef]
- Ramin, S.; Weller, M.G. Extremely sensitive and selective antibodies against the explosive 2,4,6-trinitrotoluene by rational design of a structurally optimized hapten. J. Mol. Recognit. 2012, 25, 89–97. [Google Scholar] [CrossRef]
- Walter, M.A.; Pfeifer, D.; Kraus, W.; Emmerling, F.; Schneider, R.J.; Panne, U.; Weller, M.G. Triacetone Triperoxide (TATP): Hapten Design and Development of Antibodies. Langmuir 2010, 26, 15418–15423. [Google Scholar] [CrossRef]
- Walter, M.A.; Panne, U.; Weller, M.G. A Novel Immunoreagent for the Specific and Sensitive Detection of the Explosive Triacetone Triperoxide (TATP). Biosensors 2011, 1, 93–106. [Google Scholar] [CrossRef] [Green Version]
- Anderson, G.P.; Goldman, E.R. TNT detection using llama antibodies and a two-step competitive fluid array immunoassay. J. Immunol. Methods 2008, 339, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ulaeto, D.O.; Hutchinson, A.P.; Nicklin, S. Sub-Nanogram Detection of RDX Explosive by Monoclonal Antibodies. Monoclon. Antib. Immunodiagn. Immunother. 2015, 34, 225–227. [Google Scholar] [CrossRef]
- Freytag, J.W.; Dickinson, J.C.; Tseng, S.Y. A Highly Sensitive Affinity-Column-Mediated Immunometric Assay, as Exemplified by Digoxin. Clin. Chem. 1984, 30, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Freytag, J.W.; Lau, H.P.; Wadsley, J.J. Affinity-Column-Mediated Immunoenzymometric Assays—Influence of Affinity-Column Ligand and Valency of Antibody Enzyme Conjugates. Clin. Chem. 1984, 30, 1494–1498. [Google Scholar] [CrossRef] [PubMed]
- Leflar, C.C.; Freytag, J.W.; Powell, L.M.; Strahan, J.C.; Wadsley, J.J.; Tyler, C.A.; Miller, W.K. An Automated, Affinity-Column-Mediated, Enzyme-Linked Immunometric Assay for Digoxin on the Du-Pont Aca Discrete Clinical Analyzer. Clin. Chem. 1984, 30, 1809–1811. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.G.; Eremenko, A.V.; Kuhn, A.; Kurzinger, K.; Makower, A.; Scheller, F.W. Automated amplified flow immunoassay for cocaine. Anal. Chem. 1998, 70, 4624–4630. [Google Scholar] [CrossRef]
- Weller, M.G. Immunochromatographic techniques—A critical review. Fresen. J. Anal. Chem. 2000, 366, 635–645. [Google Scholar] [CrossRef]
- Oosterkamp, A.J.; Irth, H.; Herraiz, M.T.V.; Tjaden, U.R.; van der Greef, J. Theoretical concepts of online liquid chromatographic biochemical detection systems.1. Detection systems based on labelled ligands. J. Chromatogr. A 1997, 787, 27–35. [Google Scholar] [CrossRef]
- Oosterkamp, A.J.; Irth, H.; Tjaden, U.R.; van der Greef, J. Theoretical concepts of online liquid chromatographic biochemical detection systems.2. Detection systems based on labelled affinity proteins. J. Chromatogr. A 1997, 787, 37–46. [Google Scholar] [CrossRef]
- Gunaratna, P.C.; Wilson, G.S. Noncompetitive Flow-Injection Immunoassay for a Hapten, Alpha-(Difluoromethyl)Ornithine. Anal. Chem. 1993, 65, 1152–1157. [Google Scholar] [CrossRef]
- Hafner, F.T.; Kautz, R.A.; Iverson, B.L.; Tim, R.C.; Karger, B.L. Non-competitive immunoassay of small analytes at the femtomolar level by affinity probe capillary electrophoresis: Direct analysis of digoxin using a uniform-labeled scFv immunoreagent. Anal. Chem. 2000, 72, 5779–5786. [Google Scholar] [CrossRef]
- Fintschenko, Y.; Wilson, G.S. Flow injection immunoassays: A review. Mikrochim. Acta 1998, 129, 7–18. [Google Scholar] [CrossRef]
- Oates, M.R.; Clarke, W.; Zimlich, A.; Hage, D.S. Optimization and development of a high-performance liquid chromatography-based one-site immunometric assay with chemiluminescence detection. Anal. Chim. Acta 2002, 470, 37–50. [Google Scholar] [CrossRef]
- Pfaunmiller, E.L.; Anguizola, J.A.; Milanuk, M.L.; Papastavros, E.; Carter, N.; Matsuda, R.; Zheng, X.W.; Hage, D.S. Development of microcolumn-based one-site immunometric assays for protein biomarkers. J. Chromatogr. A 2014, 1366, 92–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovgren, U.; Kronkvist, K.; Backstrom, B.; Edholm, L.E.; Johansson, G. Design of non-competitive flow injection enzyme immunoassays for determination of haptens: Application to digoxigenin. J. Immunol. Methods 1997, 208, 159–168. [Google Scholar] [CrossRef]
- Danilova, N.P.; Beckman, N.I.; Yazynin, S.A.; Vasilov, R.G. Fast Filtration Enzyme-Immunoassay for Haptens. Immunol. Lett. 1992, 33, 157–162. [Google Scholar] [CrossRef]
- Ohmura, N.; Lackie, S.J.; Saiki, H. An immunoassay for small analytes with theoretical detection limits. Anal. Chem. 2001, 73, 3392–3399. [Google Scholar] [CrossRef]
- Blake, R.C.; Pavlov, A.R.; Blake, D.A. Automated kinetic exclusion assays to quantify protein binding interactions in homogeneous solution. Anal. Biochem. 1999, 272, 123–134. [Google Scholar] [CrossRef]
- Tscheuschner, G.; Schwaar, T.; Weller, M.G. Fast Confirmation of Antibody Identity by MALDI-TOF MS Fingerprints. Antibodies (Basel) 2020, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Paul, M.; Weller, M.G. Antibody Screening by Microarray Technology—Direct Identification of Selective High-Affinity Clones. Antibodies (Basel) 2020, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Sapsford, K.E.; Charles, P.T.; Patterson, C.H.; Ligler, F.S. Demonstration of four immunoassay formats using the array biosensor. Anal. Chem. 2002, 74, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, A.; Mathies, R.A. Homogeneous immunoassay for detection of TNT and its analogues on a microfabricated capillary electrophoresis chip. Anal. Chem. 2003, 75, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, A.; Mathies, R.A. Multichannel homogeneous immunoassay for detection of 2,4,6-trinitrotoluene (TNT) using a microfabricated capillary array electrophoresis chip. Electrophoresis 2004, 25, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.P.; Moreira, S.C.; Charles, P.T.; Medintz, I.L.; Goldman, E.R.; Zeinali, M.; Taitt, C.R. TNT detection using multiplexed liquid array displacement immunoassays. Anal. Chem. 2006, 78, 2279–2285. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Shankaran, D.R.; Kim, S.J.; Gobi, K.V.; Matsumoto, K.; Toko, K.; Miura, N. Fabrication of a novel immunosensor using functionalized self-assembled monolayer for trace level detection of TNT by surface plasmon resonance. Talanta 2007, 72, 554–560. [Google Scholar] [CrossRef]
- Shankaran, D.R.; Kawaguchi, T.; Kim, S.J.; Matsumoto, K.; Toko, K.; Miura, N. Evaluation of the molecular recognition of monoclonal and polyclonal antibodies for sensitive detection of 2,4,6-trinitrotoluene (TNT) by indirect competitive surface plasmon resonance immunoassay. Anal. Bioanal. Chem. 2006, 386, 1313–1320. [Google Scholar] [CrossRef]
- Anderson, G.P.; Lamar, J.D.; Charles, P.T. Development of a luminex based competitive immunoassay for 2,4,6-trinitrotoluene (TNT). Environ. Sci. Technol. 2007, 41, 2888–2893. [Google Scholar] [CrossRef]
- Girotti, S.; Eremin, S.A.; Montoya, A.; Moreno, M.J.; Caputo, P.; D’Elia, M.; Ripani, L.; Romolo, F.S.; Maiolini, E. Development of a chemiluminescent ELISA and a colloidal gold-based LFIA for TNT detection. Anal. Bioanal. Chem. 2010, 396, 687–695. [Google Scholar] [CrossRef]
- Giannetto, M.; Maiolini, E.; Ferri, E.N.; Girotti, S.; Mori, G.; Careri, M. Competitive amperometric immunosensor based on covalent linking of a protein conjugate to dendrimer-functionalised nanogold substrate for the determination of 2,4,6-trinitrotoluene. Anal. Bioanal. Chem. 2013, 405, 737–743. [Google Scholar] [CrossRef]
- Wang, J.; AlMakhaita, M.; Biswal, S.L.; Segatori, L. Sensitive Detection of TNT using Competition Assay on Quartz Crystal Microbalance. J. Biosens. Bioelectron. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Charles, P.T.; Wadhwa, V.; Kouyate, A.; Mesa-Donado, K.J.; Adams, A.A.; Deschamps, J.R.; Kusterbeck, A.W. A High Aspect Ratio Bifurcated 128-Microchannel Microfluidic Device for Environmental Monitoring of Explosives. Sensors 2018, 18, 1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yatabe, R.; Onodera, T.; Toko, K. Fabrication of an SPR Sensor Surface with Antifouling Properties for Highly Sensitive Detection of 2,4,6-Trinitrotoluene Using Surface-Initiated Atom Transfer Polymerization. Sensors (Basel) 2013, 13, 9294–9304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuta, Y.; Onodera, T.; Singh, P.; Matsumoto, K.; Miura, N.; Toko, K. Development of an oligo(ethylene glycol)-based SPR immunosensor for TNT detection. Biosens. Bioelectron. 2008, 24, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Onodera, T.; Mizuta, Y.; Matsumoto, K.; Miura, N.; Toko, K. Novel DNP-KLH protein conjugate surface for sensitive detection of TNT on SPR immunosensor. Sens. Mater. 2007, 19, 261–273. [Google Scholar]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, M.; Tscheuschner, G.; Herrmann, S.; Weller, M.G. Fast Detection of 2,4,6-Trinitrotoluene (TNT) at ppt Level by a Laser-Induced Immunofluorometric Biosensor. Biosensors 2020, 10, 89. https://doi.org/10.3390/bios10080089
Paul M, Tscheuschner G, Herrmann S, Weller MG. Fast Detection of 2,4,6-Trinitrotoluene (TNT) at ppt Level by a Laser-Induced Immunofluorometric Biosensor. Biosensors. 2020; 10(8):89. https://doi.org/10.3390/bios10080089
Chicago/Turabian StylePaul, Martin, Georg Tscheuschner, Stefan Herrmann, and Michael G. Weller. 2020. "Fast Detection of 2,4,6-Trinitrotoluene (TNT) at ppt Level by a Laser-Induced Immunofluorometric Biosensor" Biosensors 10, no. 8: 89. https://doi.org/10.3390/bios10080089
APA StylePaul, M., Tscheuschner, G., Herrmann, S., & Weller, M. G. (2020). Fast Detection of 2,4,6-Trinitrotoluene (TNT) at ppt Level by a Laser-Induced Immunofluorometric Biosensor. Biosensors, 10(8), 89. https://doi.org/10.3390/bios10080089