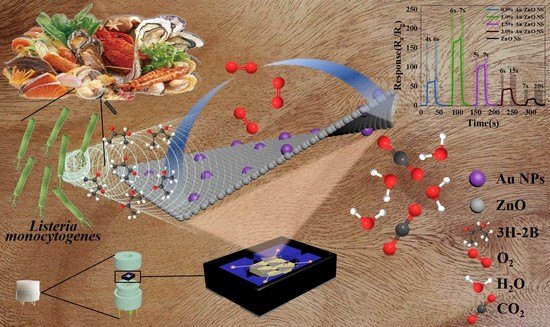
Enhanced Response for Foodborne Pathogens Detection by Au Nanoparticles Decorated ZnO Nanosheets Gas Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Synthesis of ZnO NS
2.4. Synthesis of Au NPs
2.5. Synthesis of Au/ZnO NS
2.6. Preparation of the Sensors
3. Results
3.1. Materials Characterization
3.2. Gas-Sensing Properties
3.3. Gas-Sensing Mechanism
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Noordhout, C.M.; Devleesschauwer, B.; Angulo, F.J.; Verbeke, G.; Haagsma, J.; Kirk, M.; Havelaar, A.; Speybroeck, N. The global burden of Listeriosis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 1073–1082. [Google Scholar] [CrossRef]
- Radoshevich, L.; Cossart, P. Listeria monocytogenes: Towards a complete picture of its physiology and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, V.; Arshak, K.; Korostynska, O.; Oliwa, K.; Adley, C. An overview of foodborne pathogen detection in the perspective of biosensors. Biotechnol. Adv. 2010, 28, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Välimaa, A.L.; Tilsala-Timisjärvi, A.; Virtanen, E. Rapid detection and identification methods for Listeria monocytogenes in the food chain—A review. Food Control 2015, 55, 103–114. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Zheng, L.J.; Zheng, S.Z.; Chen, J.; Liang, M.H.; Tian, Y.T.; Yang, D.C. Cr doped WO3 nanofibers enriched with surface oxygen vacancies for highly sensitive detection of the 3-hydroxy-2-butanone biomarker. J. Mater. Chem. A 2018, 6, 21419–21427. [Google Scholar] [CrossRef]
- Yu, Y.X.; Sun, X.H.; Liu, Y.; Pan, Y.J.; Zhao, Y. Odor fingerprinting of Listeria monocytogenes recognized by SPME–GC–MS and E-nose. Can. J. Microbiol. 2015, 61, 367–372. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Zhao, Y.; Ma, J.H.; Cheng, X.W.; Xie, J.; Xu, P.C.; Liu, H.Q.; Liu, H.P.; Zhang, H.J.; Wu, M.H.; et al. Mesoporous tungsten oxides with crystalline framework for highly sensitive and selective detection of foodborne pathogens. J. Am. Chem. Soc. 2017, 139, 10365–10373. [Google Scholar] [CrossRef]
- Cai, H.J.; Liu, H.Q.; Ni, T.J.; Pan, Y.J.; Zhao, Y.; Zhu, Y.H. Controlled synthesis of Pt doped SnO2 mesoporous hollow nanospheres for highly selective and rapidly detection of 3-hydroxy-2-butanone biomarker. Front. Chem. 2019, 7, 843. [Google Scholar] [CrossRef]
- Xu, D.P.; Ge, K.J.; Qi, S.Y.; Chen, Y.; Qiu, J.X.; Liu, Q. Hydrangea-like mesoporous WO3 nanoflowers with crystalline framework for 3-hydroxy-2-butanone sensing. Anal. Bioanal. Chem. 2020, 412, 8371–8378. [Google Scholar] [CrossRef]
- Xing, X.X.; Zhu, Z.Y.; Du, L.L.; Feng, D.L.; Chen, J.; Li, S.; Yang, D.C. Burrs-shelled SnO2@Al2O3 nanocables for detection of 3-hydroxy-2-butanone biomarkers. Appl. Surf. Sci. 2020, 502, 144106. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Z.Y.; Zheng, S.Z.; Du, L.L.; Xing, X.X.; Feng, D.L.; Li, S.; Yang, D.C. Synthesis of zinc oxide-alumina nanocables for detection of 3-hydroxy-2-butanone biomarker. Mater. Lett. 2019, 253, 121–123. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Zheng, L.J.; Zheng, S.Z.; Chen, J.; Xing, X.; Feng, D.L.; Yang, D.C. Multichannel pathway-enriched mesoporous NiO nanocuboids for the highly sensitive and selective detection of 3-hydroxy-2-butanone biomarkers. J. Mater. Chem. A 2019, 7, 10456–10463. [Google Scholar] [CrossRef]
- Xu, J.Q.; Han, J.J.; Zhang, Y.; Sun, Y.A.; Xie, B. Studies on alcohol sensing mechanism of ZnO based gas sensors. Sens. Actuators B Chem. 2009, 132, 334–339. [Google Scholar] [CrossRef]
- Meng, F.L.; Zheng, H.X.; Sun, Y.F.; Li, M.Q.; Liu, J.H. Trimethylamine sensors based on au-modified hierarchical porous single-crystalline ZnO nanosheets. Sensors 2017, 17, 1478. [Google Scholar] [CrossRef]
- Choi, M.S.; Kim, M.Y.; Mirzaei, A.; Kim, H.S.; Kim, S.I.; Baek, S.H.; Chun, D.W.; Jin, C.H.; Lee, K.H. Selective, sensitive, and stable NO2 gas sensor based on porous ZnO nanosheets. Appl. Surf. Sci. 2021, 569, 150910. [Google Scholar] [CrossRef]
- Xu, D.S.; Xu, P.C.; Wang, X.Q.; Chen, Y.; Yu, H.T.; Zheng, D.; Li, X.X. Pentagram-shaped Ag@Pt core-shell nanostructures as high-performance catalysts for formaldehyde detection. ACS Appl. Mater. Interfaces 2020, 12, 8091–8097. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, I.; Jang, B.H.; Youn, D.Y.; Ryu, W.H.; Park, C.O.; Kim, I.D. Selective diagnosis of diabetes using ptfunctionalized WO3 hemitube networks as a sensing layer of acetone in exhaled breath. Anal. Chem. 2013, 85, 1792–1796. [Google Scholar] [CrossRef]
- Jang, J.S.; Yu, S.; Choi, S.J.; Kim, S.J.; Koo, W.T.; Kim, I.D. Metal chelation assisted in situ migration and functionalization of catalysts on peapod-like hollow SnO2 toward a superior chemical sensor. Small 2016, 12, 5989–5997. [Google Scholar] [CrossRef]
- Wang, Y.L.; Cui, X.B.; Yang, Q.Y.; Liu, J.; Gao, Y.; Sun, P.; Lu, G.Y. Preparation of Ag-loaded mesoporous WO3 and its enhanced NO2 sensing performance. Sens. Actuators B Chem. 2016, 225, 544–552. [Google Scholar] [CrossRef]
- Zhao, C.; Shen, J.B.; Xu, S.S.; Wei, J.; Liu, H.Q.; Xie, S.Q.; Pan, Y.J.; Zhao, Y.; Zhu, Y.H. Ultra-efficient trimethylamine gas sensor based on Au nanoparticles sensitized WO3 nanosheets for rapid assessment of seafood freshness. Food Chem. 2022, 392, 133318. [Google Scholar] [CrossRef]
- Majhi, S.M.; Rai, P.; Yu, Y.T. Facile approach to synthesize Au@ZnO core-shell NPs and their application for highly sensitive and selective gas sensors. ACS Appl. Mater. Interfaces 2015, 7, 9462–9468. [Google Scholar] [CrossRef]
- Wang, L.W.; Wang, S.R.; Xu, M.J.; Hu, X.J.; Zhang, H.X.; Wang, Y.S.; Huang, W.P. A Au-functionalized ZnO nanowire gas sensor for detection of benzene and toluene. Phys. Chem. Chem. Phys. 2013, 15, 17179–17186. [Google Scholar] [CrossRef]
- Meng, F.L.; Ge, S.; Jia, Y.; Sun, B.; Sun, Y.F.; Wang, C.; Wu, H.; Jin, Z.; Li, M.Q. Interlaced nanoflake-assembled flower-like hierarchical ZnO microspheres prepared by bisolvents and their sensing properties to ethanol. J. Alloys Compd. 2015, 632, 645–650. [Google Scholar] [CrossRef]
- Meng, F.L.; Hou, N.N.; Ge, S.; Sun, B.; Jin, Z.; Shen, W.; Kong, L.T.; Guo, Z.; Sun, Y.F.; Wu, H.; et al. Flower-like hierarchical structures consisting of porous single-crystalline ZnO nanosheets and their gas sensing properties to volatile organic compounds (VOCs). J. Alloys Compd. 2015, 626, 124–130. [Google Scholar] [CrossRef]
- Hou, N.N.; Jin, Z.; Sun, B.; Sun, Y.F.; Shen, W.; Guo, Z.; Kong, L.T.; Li, M.Q.; Meng, F.L. New strategy for rapid detection of the simulants of persistent organic pollutants using gas sensor based on 3-D porous single-crystalline ZnO nanosheets. IEEE Sens. J. 2015, 15, 3668–3674. [Google Scholar] [CrossRef]
- Han, T.; Zhang, Y.; Xu, J.; Dong, J.; Liu, C.C. Monodisperse AuM (M = Pd, Rh, Pt) bimetallic nanocrystals for enhanced electrochemical detection of H2O2. Sens. Actuators B Chem. 2015, 207, 404–412. [Google Scholar] [CrossRef]
- Shen, J.B.; Xu, S.S.; Zhao, C.; Qiao, X.P.; Liu, H.Q.; Zhao, Y.; Wei, J.; Zhu, Y.H. Bimetallic Au@Pt nanocrystal sensitization mesoporous alpha-Fe2O3 hollow nanocubes for highly sensitive and rapid detection of fish freshness at low temperature. ACS Appl. Mater. Interfaces 2021, 13, 57597–57608. [Google Scholar] [CrossRef]
- Wang, D.; Deng, L.F.; Cai, H.J.; Yang, J.L.; Bao, L.P.; Zhu, Y.H.; Wang, X.Y. Bimetallic PtCu nanocrystal sensitization WO3 hollow spheres for highly efficient 3-hydroxy-2-butanone biomarker detection. ACS Appl. Mater. Interfaces 2020, 12, 18904–18912. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, F.; Zhu, C.Q.; Li, Q.; Song, J.N.; Zheng, M.J.; Ma, L.; Shen, W.Z. A facile self-assembly synthesis of hexagonal ZnO nanosheet films and their photoelectrochemical properties. Nano-Micro Lett. 2016, 8, 137–142. [Google Scholar] [CrossRef]
- Huang, M.H.; Wu, Y.Y.; Feick, H.; Tran, N.; Weber, E.; Yang, P.D. Catalytic growth of zinc oxide nanowires by vapor transport. Adv. Mater. 2001, 13, 113–116. [Google Scholar] [CrossRef]
- Li, G.; Cheng, Z.; Xiang, Q.; Yan, L.; Wang, X.; Xu, J. Bimetal PdAu decorated SnO2 nanosheets based gas sensor with temperature-dependent dual selectivity for detecting formaldehyde and acetone. Sens. Actuators B Chem. 2019, 283, 590–601. [Google Scholar] [CrossRef]
- Song, X.; Xu, Q.; Zhang, T.; Song, B.; Li, C.; Cao, B. Room-temperature, high selectivity and low-ppm-level triethylamine sensor assembled with au decahedrons-decorated porous α-Fe2O3 nanorods directly grown on flat substrate. Sens. Actuators B Chem. 2018, 268, 170–181. [Google Scholar] [CrossRef]
- Cao, P.J.; Huang, Q.G.; Navale, S.T.; Fang, M.; Liu, X.K.; Zeng, Y.X.; Liu, W.J.; Stadler, F.J.; Lu, Y.M. Integration of, esoporous ZnO and Au@ZnO nanospheres into sensing device for the ultrasensitive CH3COCH3 detection down to ppb levels. Appl. Surf. Sci. 2020, 518, 146223–146234. [Google Scholar] [CrossRef]
- Pelicano, C.M.; Yanagi, H. Accelerated growth of nanostructured ZnO films via low temperature microwave-assisted H2O oxidation for solar cell applications. Appl. Surf. Sci. 2020, 506, 144917. [Google Scholar] [CrossRef]
- Ma, J.H.; Ren, Y.; Zhou, X.R.; Liu, L.L.; Zhu, Y.H.; Cheng, X.W.; Xu, P.C.; Li, X.X.; Deng, Y.H.; Zhao, D.Y. Pt nanoparticles sensitized ordered mesoporous WO3 semiconductor: Gas sensing performance and mechanism study. Adv. Funct. Mater. 2018, 28, 1705268. [Google Scholar] [CrossRef]
- Chen, J.; Feng, D.L.; Wang, C.; Xing, X.X.; Du, L.L.; Zhu, Z.Y.; Huang, X.H.; Yang, D.C. Gas sensor detecting 3-hydroxy-2-butanone biomarkers: Boosted response via decorating Pd nanoparticles onto the {010} facets of BiVO4 decahedrons. ACS Sens. 2020, 5, 2620–2627. [Google Scholar] [CrossRef]
- Lee, J.H.; Mirzaei, A.; Kim, J.Y.; Kim, J.H.; Kim, H.W.; Kim, S.S. Optimization of the surface coverage of metal nanoparticles on nanowires gas sensors to achieve the optimal sensing performance. Sens. Actuators B Chem. 2019, 302, 127196. [Google Scholar] [CrossRef]
- Wan, L.; Song, H.Y.; Ma, J.H.; Ren, Y.; Cheng, X.W.; Su, J.C.; Yue, Q.; Deng, Y.H. Polymerization-induced colloid assembly route to iron oxide-based mesoporous microspheres for gas sensing and fenton catalysis. ACS Appl. Mater. Interfaces 2018, 10, 13028–13039. [Google Scholar] [CrossRef]
- Xiao, X.Y.; Liu, L.L.; Ma, J.H.; Ren, Y.; Cheng, X.W.; Zhu, Y.H.; Zhao, D.Y.; Elzatahry, A.A.; Alghamdi, A.; Deng, Y.H. Ordered mesoporous tin oxide semiconductors with large pores and crystallized walls for high-performance gas sensing. ACS Appl. Mater. Interfaces 2018, 10, 1871–1880. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Zhao, P.; Li, W.; Wang, Q.; Sun, P.; Chuai, X.; Lu, G. Porous alpha-Fe2O3 microflowers: Synthesis, structure, and enhanced acetone sensing performances. J. Colloid Interface Sci. 2017, 505, 1039–1046. [Google Scholar] [CrossRef]
- Zhang, F.D.; Dong, X.; Cheng, X.L.; Xu, Y.M.; Zhang, X.F.; Huo, L.H. Enhanced gas-sensing properties for trimethylamine at low temperature based on MoO3/Bi2Mo3O12 hollow microspheres. ACS Appl. Mater. Interfaces 2019, 11, 11755–11762. [Google Scholar] [CrossRef]
- Liu, C.; Gao, H.; Wang, L.; Wang, T.; Yang, X.; Sun, P.; Gao, Y.; Liang, X.; Liu, F.; Song, H.; et al. Facile synthesis and the enhanced sensing properties of Pt-loaded α-Fe2O3 porous nanospheres. Sens. Actuators B Chem. 2017, 252, 1153–1162. [Google Scholar] [CrossRef]
- Cai, H.J.; Qiao, X.P.; Chen, M.L.; Feng, D.S.; Alghamdi, A.A.; Alharthi, F.A.; Pan, Y.J.; Zhao, Y.; Zhu, Y.H.; Deng, Y.H. Hydrothermal synthesis of hierarchical SnO2 nanomaterials for high-efficiency detection of pesticide residue. Chin. Chem. Lett. 2021, 32, 1502–1506. [Google Scholar] [CrossRef]
- Chen, Y.P.; Li, Y.X.; Feng, B.X.; Wu, Y.; Zhu, Y.H.; Wei, J. Self-templated synthesis of mesoporous Au-ZnO nanospheres for seafood freshness detection. Sens. Actuators B Chem. 2022, 360, 131662. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, W.M.; Liu, C.; Foda, M.F.; Zhu, Y.H. Strawberry-like SiO2/Ag nanocomposites immersed filter paper as SERS substrate for acrylamide detection. Food Chem. 2020, 328, 127106. [Google Scholar] [CrossRef]
- Wu, L.; Xianyu, Y.L.; Wang, Z.L.; Dong, Y.Z.; Hu, X.B.; Chen, Y.P. Amplified magnetic resonance sensing via enzyme-mediated click chemistry and magnetic separation. Anal. Chem. 2020, 91, 15555–15562. [Google Scholar] [CrossRef]
- Sakai, G.; Matsunaga, N.; Shimanoe, K.; Yamazoe, N. Theory of gas-diffusion controlled sensitivity for thin film semiconductor gas sensor. Sens. Actuators B Chem. 2001, 80, 125–131. [Google Scholar] [CrossRef]
- Wang, L.L.; Dou, H.M.; Lou, Z.; Zhang, T. Encapsuled nanoreactors (Au@SnO2): A new sensing material for chemical sensors. Nanoscale 2013, 5, 2686–2691. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Xu, S.; Wei, J.; Xie, S.; Wei, J.; Han, J.; Zhang, Z.; Liu, H.; Cheng, J.; Zhao, Y.; et al. Enhanced Response for Foodborne Pathogens Detection by Au Nanoparticles Decorated ZnO Nanosheets Gas Sensor. Biosensors 2022, 12, 803. https://doi.org/10.3390/bios12100803
Zhao C, Xu S, Wei J, Xie S, Wei J, Han J, Zhang Z, Liu H, Cheng J, Zhao Y, et al. Enhanced Response for Foodborne Pathogens Detection by Au Nanoparticles Decorated ZnO Nanosheets Gas Sensor. Biosensors. 2022; 12(10):803. https://doi.org/10.3390/bios12100803
Chicago/Turabian StyleZhao, Cheng, Shanshan Xu, Jing Wei, Siqi Xie, Jinlei Wei, Jingting Han, Zhaohuan Zhang, Haiquan Liu, Jinsheng Cheng, Yong Zhao, and et al. 2022. "Enhanced Response for Foodborne Pathogens Detection by Au Nanoparticles Decorated ZnO Nanosheets Gas Sensor" Biosensors 12, no. 10: 803. https://doi.org/10.3390/bios12100803
APA StyleZhao, C., Xu, S., Wei, J., Xie, S., Wei, J., Han, J., Zhang, Z., Liu, H., Cheng, J., Zhao, Y., & Zhu, Y. (2022). Enhanced Response for Foodborne Pathogens Detection by Au Nanoparticles Decorated ZnO Nanosheets Gas Sensor. Biosensors, 12(10), 803. https://doi.org/10.3390/bios12100803