Leveraging Accelerometry as a Prognostic Indicator for Increase in Opioid Withdrawal Symptoms
Abstract
:1. Introduction
2. Materials and Methods
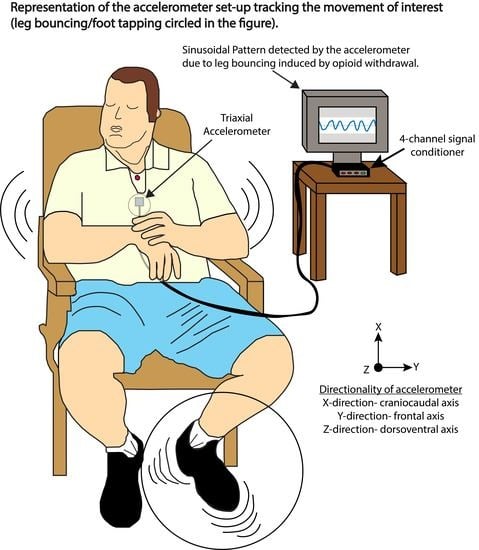
2.1. Patient Study Sample and Assessments
2.2. Creating Spectrograms from the Accelerometer Data
2.3. Processing the Accelerometer Data to Extract Frequency and Maximum Power Spectral Density
2.4. Analysis Methods of the Accelerometer Data
3. Results
3.1. Classification of Patients as “Sinusoidal” vs. “Non-Sinusoidal”
3.2. Significant Correlations Are Typically Found in the LOC
3.3. Demographic Prognostic Indicators of Sinusoidal and Non-Sinusoidal Accelerometer Waveforms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NIDA. Overdose Death Rates. Available online: https://www.drugabuse.gov/drug-topics/trends-statistics/overdose-death-rates (accessed on 22 November 2021).
- Charney, D.S.; Heninger, G.R.; Kleber, H.D. The combined use of clonidine and naltrexone as a rapid, safe, and effective treatment of abrupt withdrawal from methadone. Am. J. Psychiatry 1986, 143, 831–837. [Google Scholar] [CrossRef]
- Mattick, R.P.; Breen, C.; Kimber, J.; Davoli, M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst. Rev. 2009, 2009, CD002209. [Google Scholar] [CrossRef]
- Beetham, B.T.; Saloner, B.; Wakeman, S.E.; Gaye, M.M.; Barnett, M.L. Access to office-based buprenorphine treatment in areas with high rates of opioid-related mortality. Ann. Intern. Med. 2019, 171, 1–9. [Google Scholar] [CrossRef]
- Canli, T.; Zhao, Z.; Brewer, J.; Gabrieli, J.D.E.; Cahill, L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. J. Neurosci. 2000, 20, RC99. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Fowler, J.S.; Wang, G.-J.; Swanson, J.M. Dopamine in drug abuse and addiction: Results from imaging studies and treatment implications. Mol. Psychiatry 2004, 9, 557–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SAMHSA. Medications for Opioid Use Disorder: For Healthcare and Addiction Professionals, Policymakers, Patients, and Families [Internet]; (Treatment Improvement Protocol (TIP) Series, No. 63); Substance Abuse and Mental Health Services Administration (US): Rockville, MD, USA, 2018. [Google Scholar]
- Lee, J.D.; Nunes, E.V.; Novo, P.; Bachrach, K.; Bailey, G.L.; Bhatt, S.; Farkas, S.; Fishman, M.; Gauthier, P.; Hodgkins, C.C.; et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): A multicentre, open-label, randomised controlled trial. Lancet 2018, 391, 309–318. [Google Scholar] [CrossRef]
- SAMHSA. Naltrexone. Available online: https://www.samhsa.gov/medication-assisted-treatment/medications-counseling-related-conditions/naltrexone (accessed on 22 November 2021).
- Mannelli, P.; Patkar, A.A.; Peindl, K.; Gorelick, D.A.; Wu, L.-T.; Gottheil, E. Very low dose naltrexone addition in opioid detoxification: A randomized, controlled trial. Addict. Biol. 2009, 14, 204–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wesson, D.R.; Ling, W. The clinical opiate withdrawal scale (COWS). J. Psychoact. Drugs 2003, 35, 253–259. [Google Scholar] [CrossRef]
- Rosenman, R.; Tennekoon, V.; Hill, L.G. Measuring bias in self-reported data. Int. J. Behav. Health Res. 2011, 2, 320–332. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, E.; Schofield, S. Cognitive bias in clinical medicine. J. R. Coll. Physicians Edinb. 2018, 48, 225–232. [Google Scholar] [CrossRef]
- Althubaiti, A. Information bias in health research: Definition, pitfalls, and adjustment methods. J. Multidiscip. Healthc. 2016, 9, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellbery, C. Flaws in clinical reasoning: A common cause of diagnostic error. Am. Fam. Physician 2011, 84, 1042–1048. [Google Scholar] [PubMed]
- Nuamah, J.K.; Sasangohar, F.; Erraguntla, M.; Mehta, R.K. The past, present and future of opioid withdrawal assessment: A scoping review of scales and technologies. BMC Med. Inform. Decis. Mak. 2019, 19, 113. [Google Scholar] [CrossRef] [Green Version]
- Park, E.J.; Park, Y.-M. Opioid withdrawal and restless legs syndrome. Chronobiol. Med. 2020, 2, 137–140. [Google Scholar] [CrossRef]
- Carreiro, S.; Smelson, D.; Ranney, M.; Horvath, K.J.; Picard, R.W.; Boudreaux, E.; Hayes, R.; Boyer, E.W. Real-time mobile detection of drug use with wearable biosensors: A pilot study. J. Med. Toxicol. 2014, 11, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carreiro, S.; Wittbold, K.; Indic, P.; Fang, H.; Zhang, J.; Boyer, E.W. Wearable biosensors to detect physiologic change during opioid use. J. Med. Toxicol. 2016, 12, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, S.; Fang, H.; Wang, H.; Carreiro, S.; Boyer, E. Automatic detection of opioid intake using wearable biosensor. In 2018 International Conference on Computing, Networking and Communications (ICNC), Maui, HI, USA, 5–8 March 2018; IEEE: Piscataway, NJ, USA, 2008; Volume 2018, pp. 784–788. [Google Scholar] [CrossRef]
- Downey, L.A.; Tysse, B.; Ford, T.; Samuels, A.C.; Wilson, R.P.; Parrott, A.C. Psychomotor Tremor and proprioceptive control problems in current and former stimulant drug users: An accelerometer study of heavy users of amphetamine, MDMA, and other recreational stimulants. J. Clin. Pharmacol. 2017, 57, 1330–1337. [Google Scholar] [CrossRef]
- Flavel, S.C.; Koch, J.D.; White, J.M.; Todd, G. Illicit stimulant use in humans is associated with a long-term increase in tremor. PLoS ONE 2012, 7, e52025. [Google Scholar] [CrossRef] [Green Version]
- Bauer, L.O. Motoric signs of CNS dysfunction associated with alcohol and cocaine withdrawal. Psychiatry Res. 1993, 47, 69–77. [Google Scholar] [CrossRef]
- Bertz, J.W.; Epstein, D.H.; Reamer, D.; Kowalczyk, W.; Phillips, K.A.; Kennedy, A.P.; Jobes, M.L.; Ward, G.; Plitnick, B.A.; Figueiro, M.G.; et al. Sleep reductions associated with illicit opioid use and clinic-hour changes during opioid agonist treatment for opioid dependence: Measurement by electronic diary and actigraphy. J. Subst. Abus. Treat. 2019, 106, 43–57. [Google Scholar] [CrossRef]
- Hartwell, E.E.; Pfeifer, J.G.; McCauley, J.L.; Maria, M.M.-S.; Back, S.E. Sleep disturbances and pain among individuals with prescription opioid dependence. Addict. Behav. 2014, 39, 1537–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, J.A.; Purple, R.; Cole, P.; Zaiwalla, Z.; Wulff, K.; Pattinson, K.T.S. Sleep disturbance in patients taking opioid medication for chronic back pain. Anaesthesia 2016, 71, 1296–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, M.D.; Kurth, M.E.; Anderson, B.J.; Blevins, C.E. A pilot crossover trial of sleep medications for sleep-disturbed methadone maintenance patients. J. Addict. Med. 2020, 14, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lewis, B.; Chapman, B.; Carreiro, S.; Venkatasubramanian, K. A machine learning-based approach for collaborative non-adherence detection during opioid abuse surveillance using a wearable biosensor. Biomed. Eng. Syst. Technol. Int. Jt. Conf. BIOSTEC Revis. Sel. Pap. 2019, 5, 310–318. [Google Scholar] [CrossRef]
- Kulbeth, H.J.; Fukuda, S.; Brents, L.K. Automated quantification of opioid withdrawal in neonatal rat pups using Ethovision® XT software. Neurotoxicol. Teratol. 2021, 84, 106959. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishna, U.; Nath, S.K.; Vishweswaraiah, S.; Uppala, L.V.; Forray, A.; Muvvala, S.B.; Mishra, N.K.; Southekal, S.; Guda, C.; Govindamangalam, H.; et al. Maternal opioid use disorder: Placental transcriptome analysis for neonatal opioid withdrawal syndrome. Genomics 2021, 113, 3610–3617. [Google Scholar] [CrossRef]
- Radhakrishna, U.; Vishweswaraiah, S.; Uppala, L.V.; Szymanska, M.; Macknis, J.; Kumar, S.; Saleem-Rasheed, F.; Aydas, B.; Forray, A.; Muvvala, S.B.; et al. Placental DNA methylation profiles in opioid-exposed pregnancies and associations with the neonatal opioid withdrawal syndrome. Genomics 2021, 113, 1127–1135. [Google Scholar] [CrossRef]
- Kulman, E.; Venkatasubramanian, K.; Chapman, B.; Carreiro, S. Identifying opioid withdrawal using wearable biosensors. Proc. Annu. Hawaii Int. Conf. Syst. Sci. 2021, 54, 3583–3592. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7871978/ (accessed on 6 December 2021). [CrossRef]
- Chintha, K.K.; Indic, P.; Chapman, B.; Boyer, E.W.; Carreiro, S. Wearable biosensors to evaluate recurrent opioid toxicity after naloxone administration: A hilbert transform approach. Proc. Annu. Hawaii Int. Conf. Syst. Sci. 2018, 2018, 3247–3252. [Google Scholar] [CrossRef]
- Senaratne, H.; Ellis, K.; Oviatt, S.; Melvin, G. Detecting and differentiating leg bouncing behaviour from everyday movements using tri-axial accelerometer data. In Proceedings of the 2020 ACM International Joint Conference on Pervasive and Ubiquitous Computing and the 2020 ACM International Symposium on Wearable Computers, Virtual Event, Cancún, Mexico, 12–17 September 2020. [Google Scholar] [CrossRef]
- Gazi, A.H.; Harrison, A.B.; Lambert, T.P.; Obideen, M.; Alavi, P.; Murrah, N.; Shallenberger, L.; Driggers, E.G.; Ortega, R.A.; Washington, B.P.; et al. Transcutaneous cervical vagus nerve stimulation reduces behavioral and physiological manifestations of withdrawal in patients with opioid use disorder: A double-blind, randomized, sham-controlled pilot study. Brain Stimul. 2022, 15, 1206–1214. [Google Scholar] [CrossRef]
- Hasin, D.S.; O’Brien, C.P.; Auriacombe, M.; Borges, G.; Bucholz, K.; Budney, A.; Compton, W.M.; Crowley, T.; Ling, W.; Petry, N.M.; et al. DSM-5 Criteria for Substance Use Disorders: Recommendations and Rationale. Am. J. Psychiatry 2013, 170, 834–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sevim, S.; Dogu, O.; Kaleagasi, H.; Aral, M.; Metin, O.; Camdeviren, H. Correlation of anxiety and depression symptoms in patients with restless legs syndrome: A population based survey. J. Neurol. Neurosurg. Psychiatry 2004, 75, 226–230. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1738920/ (accessed on 20 December 2021). [PubMed]
- Scholz, H.; Benes, H.; Happe, S.; Bengel, J.; Kohnen, R.; Hornyak, M. Psychological distress of patients suffering from restless legs syndrome: A cross-sectional study. Health Qual. Life Outcomes 2011, 9, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beylergil, S.B.; Singh, A.P.; Zee, D.S.; Jinnah, H.A.; Shaikh, A.G. Relationship between jerky and sinusoidal oscillations in cervical dystonia. Park. Relat. Disord. 2019, 66, 130–137. [Google Scholar] [CrossRef]
- Dirkx, M.F.; Ouden, H.E.M.D.; Aarts, E.; Timmer, M.H.M.; Bloem, B.R.; Toni, I.; Helmich, R. Dopamine controls Parkinson’s tremor by inhibiting the cerebellar thalamus. Brain 2017, 140, 721–734. [Google Scholar] [CrossRef] [Green Version]
- Deik, A.; Saunders-Pullman, R.; Luciano, M.S. Substance abuse and movement disorders: Complex Interactions and comorbidities. Curr. Drug Abus. Rev. 2012, 5, 243–253. [Google Scholar] [CrossRef]
- Wei, C.; Han, X.; Weng, D.; Feng, Q.; Qi, X.; Li, J.; Luo, M. Response dynamics of midbrain dopamine neurons and serotonin neurons to heroin, nicotine, cocaine, and MDMA. Cell Discov. 2018, 4, 60. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Guidelines for Withdrawal Management and Treatment of Drug Dependence in Closed Settings; World Health Organization: Geneva, Switzerland, 2009.
- Brady, K.T.; Haynes, L.F.; Hartwell, K.J.; Killeen, T.K. Substance use disorders and anxiety: A treatment challenge for social workers. Soc. Work Public Health 2013, 28, 407–423. [Google Scholar] [CrossRef]
- Lopez, W.; Jeste, D.V. Movement disorders and substance abuse. Psychiatr. Serv. 1997, 48, 634–636. [Google Scholar] [CrossRef]
- Meina, M.; Ratajczak, E.; Sadowska, M.; Rykaczewski, K.; Dreszer, J.; Bałaj, B.; Biedugnis, S.; Węgrzyński, W.; Krasuski, A. Heart Rate variability and accelerometry as classification tools for monitoring perceived stress levels—A pilot study on firefighters. Sensors 2020, 20, 2834. [Google Scholar] [CrossRef]
- Landreani, F.; Faini, A.; Martin-Yebra, A.; Morri, M.; Parati, G.; Caiani, E.G. Assessment of ultra-short heart variability indices derived by smartphone accelerometers for stress detection. Sensors 2019, 19, 3729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Can, Y.S.; Chalabianloo, N.; Ekiz, D.; Ersoy, C. Continuous stress detection using wearable sensors in real life: Algorithmic programming contest case study. Sensors 2019, 19, 1849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantsch, J.R.; Baker, D.; Funk, D.; Lê, A.D.; Shaham, Y. Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology 2015, 41, 335–356. [Google Scholar] [CrossRef] [PubMed]




| N | Δ COWS Scores a | Normalized Max PSD b | Pearson’s R | p-Value | |
|---|---|---|---|---|---|
| FOC c | 5 | 5.40 ± 3.29 | 0.11 ± 0.02 | 0.20 | 0.74 |
| LOC d | 5 | 5.60 ± 3.05 | 0.11 ± 0.02 | 0.92 | 0.03 |
| N | Frequency (Hz) | Normalized Max PSD | Pearson’s R | p-Value | |
|---|---|---|---|---|---|
| All Patients | 7 | 4.48 ± 1.37 | 0.11 ± 0.02 | 0.69 | 0.09 |
| Det. Only | 5 | 3.39 ± 1.12 | 0.11 ± 0.02 | 0.96 | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambert, T.P.; Gazi, A.H.; Harrison, A.B.; Gharehbaghi, S.; Chan, M.; Obideen, M.; Alavi, P.; Murrah, N.; Shallenberger, L.; Driggers, E.G.; et al. Leveraging Accelerometry as a Prognostic Indicator for Increase in Opioid Withdrawal Symptoms. Biosensors 2022, 12, 924. https://doi.org/10.3390/bios12110924
Lambert TP, Gazi AH, Harrison AB, Gharehbaghi S, Chan M, Obideen M, Alavi P, Murrah N, Shallenberger L, Driggers EG, et al. Leveraging Accelerometry as a Prognostic Indicator for Increase in Opioid Withdrawal Symptoms. Biosensors. 2022; 12(11):924. https://doi.org/10.3390/bios12110924
Chicago/Turabian StyleLambert, Tamara P., Asim H. Gazi, Anna B. Harrison, Sevda Gharehbaghi, Michael Chan, Malik Obideen, Parvaneh Alavi, Nancy Murrah, Lucy Shallenberger, Emily G. Driggers, and et al. 2022. "Leveraging Accelerometry as a Prognostic Indicator for Increase in Opioid Withdrawal Symptoms" Biosensors 12, no. 11: 924. https://doi.org/10.3390/bios12110924
APA StyleLambert, T. P., Gazi, A. H., Harrison, A. B., Gharehbaghi, S., Chan, M., Obideen, M., Alavi, P., Murrah, N., Shallenberger, L., Driggers, E. G., Alvarado Ortega, R., Washington, B., Walton, K. M., Tang, Y. -L., Gupta, R., Nye, J. A., Welsh, J. W., Vaccarino, V., Shah, A. J., ... Inan, O. T. (2022). Leveraging Accelerometry as a Prognostic Indicator for Increase in Opioid Withdrawal Symptoms. Biosensors, 12(11), 924. https://doi.org/10.3390/bios12110924