A Hybrid Titanium-Softmaterial, High-Strength, Transparent Cranial Window for Transcranial Injection and Neuroimaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Fabrication of Titanium Alloy Frames
2.2. Fabrication of the Hybrid Ti-PDMS Transparent Cranial Windows
2.3. Characterization of Hybrid Ti-PDMS Cranial Windows
2.3.1. Morphology and Hydrophobic Characterization
2.3.2. Mechanical Characterization
2.3.3. Optical Characterization
2.4. Animals and Housing
2.5. Histological Staining
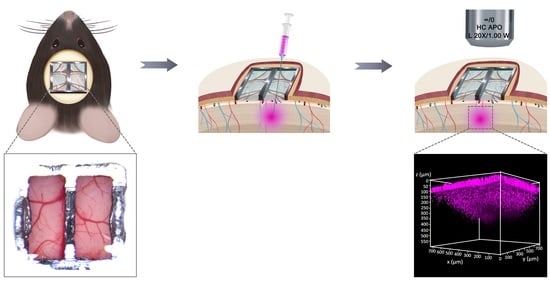
2.6. Ti-PDMS Cranial Window Implantation in Mice
2.7. Ti-PDMS Cranial Window Penetration, Dye Injection and Imaging
3. Results and Discussions
3.1. 3D Printed Ti Supporting Frames with Different Size and Shape
3.2. Morphology and Transparency of Hybrid Ti-PDMS Cranial Window
3.3. Proportion of Transparent Area and Mechanical Properties of Ti-PDMS Device
3.4. Optical Properties of Transparent Ti-PDMS Cranial Window
3.5. Implantation and Functional Evaluation of Chronic Ti-PDMS Cranial Window
3.6. Multi-Site Injection and In Vivo Two-Photon Imaging through Ti-PDMS Cranial Window
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buckner, R.L.; Krienen, F.M.; Yeo, B.T. Opportunities and limitations of intrinsic functional connectivity MRI. Nat. Neurosci. 2013, 16, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.-N.; Xing, X.-X. Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: A systems neuroscience perspective. Neurosci. Biobehav. Rev. 2014, 45, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Sepede, G.; De Berardis, D.; Campanella, D.; Perrucci, M.G.; Ferretti, A.; Serroni, N.; Moschetta, F.S.; Del Gratta, C.; Salerno, R.M.; Ferro, F.M.; et al. Impaired sustained attention in euthymic bipolar disorder patients and non-affected relatives: An fMRI study. Bipolar Disord. 2012, 14, 764–779. [Google Scholar] [CrossRef] [PubMed]
- Ebisch, S.J.H.; Salone, A.; Ferri, F.; De Berardis, D.; Romani, G.L.; Ferro, F.M.; Gallese, V. Out of touch with reality? Social perception in first-episode schizophrenia. Soc. Cogn. Affect. Neurosci. 2012, 8, 394–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boto, E.; Holmes, N.; Leggett, J.; Roberts, G.; Shah, V.; Meyer, S.S.; Muñoz, L.D.; Mullinger, K.J.; Tierney, T.M.; Bestmann, S.; et al. Moving magnetoencephalography towards real-world applications with a wearable system. Nature 2018, 555, 657–661. [Google Scholar] [CrossRef]
- Montagne, A.; Nation, D.A.; Sagare, A.P.; Barisano, G.; Sweeney, M.D.; Chakhoyan, A.; Pachicano, M.; Joe, E.; Nelson, A.R.; D’Orazio, L.M.; et al. APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature 2020, 581, 71–76. [Google Scholar] [CrossRef]
- Sawangjai, P.; Hompoonsup, S.; Leelaarporn, P.; Kongwudhikunakorn, S.; Wilaiprasitporn, T. Consumer Grade EEG Measuring Sensors as Research Tools: A Review. IEEE Sens. J. 2020, 20, 3996–4024. [Google Scholar] [CrossRef]
- Li, P.; Yin, C.; Li, M.; Li, H.; Yang, B. A dry electroencephalogram electrode for applications in steady-state visual evoked potential-based brain–computer interface systems. Biosens. Bioelectron. 2021, 187, 113326. [Google Scholar] [CrossRef]
- Pinti, P.; Tachtsidis, I.; Hamilton, A.; Hirsch, J.; Aichelburg, C.; Gilbert, S.; Burgess, P.W. The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience. Ann. N. Y. Acad. Sci. 2020, 1464, 5–29. [Google Scholar] [CrossRef]
- Quaresima, V.; Ferrari, M. Functional Near-Infrared Spectroscopy (fNIRS) for Assessing Cerebral Cortex Function During Human Behavior in Natural/Social Situations: A Concise Review. Organ. Res. Methods 2016, 22, 46–68. [Google Scholar] [CrossRef]
- Kalchenko, V.; Israeli, D.; Kuznetsov, Y.; Meglinski, I.; Harmelin, A. A simple approach for non-invasive transcranial optical vascular imaging (nTOVI). J. Biophotonics 2015, 8, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Kalchenko, V.; Sdobnov, A.; Meglinski, I.; Kuznetsov, Y.; Molodij, G.; Harmelin, A. A Robust Method for Adjustment of Laser Speckle Contrast Imaging during Transcranial Mouse Brain Visualization. Photonics 2019, 6, 80. [Google Scholar] [CrossRef] [Green Version]
- Feng, W.; Zhang, C.; Yu, T.; Semyachkina-Glushkovskaya, O.; Zhu, D. In vivo monitoring blood-brain barrier permeability using spectral imaging through optical clearing skull window. J. Biophotonics 2019, 12, e201800330. [Google Scholar] [CrossRef] [PubMed]
- Molodij, G.; Sdobnov, A.; Kuznetsov, Y.; Harmelin, A.; Meglinski, I.; Kalchenko, V.V. Time-space Fourier κω′ filter for motion artifacts compensation during transcranial fluorescence brain imaging. Phys. Med. Biol. 2020, 65, 075007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semyachkina-Glushkovskaya, O.V.; Kurths, J.; Pavlov, A.N.; Borisova, E.G.; Abdurashitov, A.S.; Zhu, D.; Li, P.; Luo, Q.; Tuchin, V. Silent Vascular Catastrophes in the Brain in Term Newborns: Strategies for Optical Imaging. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 88–101. [Google Scholar] [CrossRef]
- Piavchenko, G.; Kozlov, I.; Dremin, V.; Stavtsev, D.; Seryogina, E.; Kandurova, K.; Shupletsov, V.; Lapin, K.; Alekseyev, A.; Kuznetsov, S.; et al. Impairments of cerebral blood flow microcirculation in rats brought on by cardiac cessation and respiratory arrest. J. Biophotonics 2021, 14, e202100216. [Google Scholar] [CrossRef]
- White-Dzuro, G.A.; Lake, W.; Eli, I.M.; Neimat, J.S. Novel Approach to Securing Deep Brain Stimulation Leads: Technique and Analysis of Lead Migration, Breakage, and Surgical Infection. Ster. Funct. Neurosurg. 2016, 94, 18–23. [Google Scholar] [CrossRef]
- Krauss, J.K.; Lipsman, N.; Aziz, T.; Boutet, A.; Brown, P.; Chang, J.W.; Davidson, B.; Grill, W.M.; Hariz, M.I.; Horn, A.; et al. Technology of deep brain stimulation: Current status and future directions. Nat. Rev. Neurol. 2021, 17, 75–87. [Google Scholar] [CrossRef]
- Homer, M.L.; Nurmikko, A.V.; Donoghue, J.P.; Hochberg, L.R. Sensors and Decoding for Intracortical Brain Computer Interfaces. Annu. Rev. Biomed. Eng. 2013, 15, 383–405. [Google Scholar] [CrossRef] [Green Version]
- Jorfi, M.; Skousen, J.L.; Weder, C.; Capadona, J.R. Progress towards biocompatible intracortical microelectrodes for neural interfacing applications. J. Neural Eng. 2015, 12, 011001. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, Z.; Cui, S.; Liu, F.-Y.; Yi, M.; Wan, Y. A Novel 3D-Printed Multi-Drive System for Synchronous Electrophysiological Recording in Multiple Brain Regions. Front. Neurosci. 2019, 13, 1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Yue, L.; Zhang, Y.; Wang, Y.; Han, B.; Cui, S.; Liu, F.-Y.; Wan, Y.; Yi, M. Spontaneous Pain Disrupts Ventral Hippocampal CA1-Infralimbic Cortex Connectivity and Modulates Pain Progression in Rats with Peripheral Inflammation. Cell Rep. 2019, 29, 1579–1593.e6. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Cui, S.; Liu, F.-Y.; Wan, Y.; Shi, Y.; Yi, M. Suppression of ventral hippocampal CA1 pyramidal neuronal activities enhances water intake. Am. J. Physiol. Physiol. 2021, 321, C992–C999. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, Y.; Yue, L.; Zhang, X.; Cui, S.; Liu, F.; Wan, Y.; Yi, M. Anterior cingulate cortex modulates the affective-motivative dimension of hyperosmolality-induced thirst. J. Physiol. 2019, 597, 4851–4860. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gu, X.-W.; Ji, B.-W.; Wang, L.-C.; Guo, Z.-J.; Yang, B.; Wang, X.-L.; Li, C.-Y.; Liu, J.-Q. Three-dimensional drivable optrode array for high-resolution neural stimulations and recordings in multiple brain regions. Biosens. Bioelectron. 2019, 131, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Fedor, F.Z.; Madarász, M.; Zátonyi, A.; Szabó, Á.; Lőrincz, T.; Danda, V.; Spurgin, L.; Manz, C.; Rózsa, B.; Fekete, Z. Soft, Thiol-ene/Acrylate-Based Electrode Array for Long-Term Recording of Intracranial EEG Signals with Improved Biocompatibility in Mice. Adv. Mater. Technol. 2021, 2100942. [Google Scholar] [CrossRef]
- Cramer, S.W.; Carter, R.E.; Aronson, J.D.; Kodandaramaiah, S.B.; Ebner, T.J.; Chen, C.C. Through the looking glass: A review of cranial window technology for optical access to the brain. J. Neurosci. Methods 2021, 354, 109100. [Google Scholar] [CrossRef]
- Zhang, Y.; Cudmore, R.; Lin, D.-T.; Linden, D.J.; Huganir, R.L. Visualization of NMDA receptor–dependent AMPA receptor synaptic plasticity in vivo. Nat. Neurosci. 2015, 18, 402–407. [Google Scholar] [CrossRef]
- Yang, G.; Pan, F.; Parkhurst, C.N.; Grutzendler, J.; Gan, W.-B. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat. Protoc. 2010, 5, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.-T.; Pan, F.; Yang, G.; Gan, W.-B. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat. Neurosci. 2007, 10, 549–551. [Google Scholar] [CrossRef]
- Holtmaat, A.; Bonhoeffer, T.; Chow, D.K.; Chuckowree, J.; De Paola, V.; Hofer, S.B.; Hübener, M.; Keck, T.; Knott, G.; Lee, W.-C.; et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 2009, 4, 1128–1144. [Google Scholar] [CrossRef] [PubMed]
- Heo, C.; Park, H.; Kim, Y.-T.; Baeg, E.; Kim, S.-G.; Suh, M. A soft, transparent, freely accessible cranial window for chronic imaging and electrophysiology. Sci. Rep. 2016, 6, 27818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Motoyama, M.; Masuda, H.; Ohta, Y.; Haruta, M.; Noda, T.; Sasagawa, K.; Tokuda, T.; Tamura, H.; Ishikawa, Y.; et al. Novel implantable imaging system for enabling simultaneous multiplanar and multipoint analysis for fluorescence potentiometry in the visual cortex. Biosens. Bioelectron. 2012, 38, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.B.; Gamarra, L.F. Getting into the brain: Liposome-based strategies for effective drug delivery across the blood–brain barrier. Int. J. Nanomed. 2016, 11, 5381–5414. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Gao, C.; Zhu, Y.; Ling, C.; Wang, Q.; Huang, Y.; Qin, J.; Wang, J.; Lu, W.; Wang, J. Natural Brain Penetration Enhancer-Modified Albumin Nanoparticles for Glioma Targeting Delivery. ACS Appl. Mater. Interfaces 2018, 10, 30201–30213. [Google Scholar] [CrossRef]
- Park, H.; You, N.; Lee, J.; Suh, M. Longitudinal study of hemodynamics and dendritic membrane potential changes in the mouse cortex following a soft cranial window installation. Neurophotonics 2019, 6, 015006. [Google Scholar] [CrossRef] [Green Version]
- Roome, C.J.; Kuhn, B. Chronic cranial window with access port for repeated cellular manipulations, drug application, and electrophysiology. Front. Cell. Neurosci. 2014, 8, 379. [Google Scholar] [CrossRef]
- Gernert, M.; Feja, M. Bypassing the Blood–Brain Barrier: Direct Intracranial Drug Delivery in Epilepsies. Pharmaceutics 2020, 12, 1134. [Google Scholar] [CrossRef]
- Goldey, G.J.; Roumis, D.K.; Glickfeld, L.L.; Kerlin, A.M.; Reid, R.C.; Bonin, V.; Schafer, D.P.; Andermann, M.L. Removable cranial windows for long-term imaging in awake mice. Nat. Protoc. 2014, 9, 2515–2538. [Google Scholar] [CrossRef]
- Han, H.; Xia, Z.; Chen, H.; Hou, C.; Li, W. Simple diffusion delivery via brain interstitial route for the treatment of cerebral ischemia. Sci. China Life Sci. 2011, 54, 235–239. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Han, H.; Du, J.; He, Q.; Jia, Y.; Yan, J.; Dai, H.; Cui, B.; Yang, J.; Wei, X.; et al. Early changes to the extracellular space in the hippocampus under simulated microgravity conditions. Sci. China Life Sci. 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Wang, W.; Wang, A.; Teng, Z.; Han, H. Brain Interstitial Fluid Drainage Alterations in Glioma-Bearing Rats. Aging Dis. 2018, 9, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Tokuno, H.; Hatanaka, N.; Chiken, S.; Ishizuka, N. An improved method with a long-shanked glass micropipette and ultrasonography for drug injection into deep brain structure of the monkey. Brain Res. Protoc. 2002, 10, 16–22. [Google Scholar] [CrossRef]
- Zuo, Y.; Lin, A.; Chang, P.; Gan, W.-B. Development of Long-Term Dendritic Spine Stability in Diverse Regions of Cerebral Cortex. Neuron 2005, 46, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.V.; Kang, S.S.; Dustin, M.L.; McGavern, D. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature 2008, 457, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Chai, Z.; Ping, X.; Song, L.-J.; Ma, C.; Ruan, Y.; Jin, X. In vivo Two-Photon Imaging Reveals Acute Cerebral Vascular Spasm and Microthrombosis After Mild Traumatic Brain Injury in Mice. Front. Neurosci. 2020, 14, 210. [Google Scholar] [CrossRef]
- Grutzendler, J.; Kasthuri, N.; Gan, W.-B. Long-term dendritic spine stability in the adult cortex. Nature 2002, 420, 812–816. [Google Scholar] [CrossRef]
- Drew, P.; Shih, A.Y.; Driscoll, J.D.; Knutsen, P.M.; Blinder, P.; Davalos, D.; Akassoglou, K.; Tsai, P.S.; Kleinfeld, D. Chronic optical access through a polished and reinforced thinned skull. Nat. Methods 2010, 7, 981–984. [Google Scholar] [CrossRef] [Green Version]
- Jeong, D.C.; Tsai, P.S.; Kleinfeld, D. All-optical osteotomy to create windows for transcranial imaging in mice. Opt. Express 2013, 21, 23160–23168. [Google Scholar] [CrossRef] [Green Version]
- Rossi, L.F.; Wykes, R.C.; Kullmann, D.M.; Carandini, M. Focal cortical seizures start as standing waves and propagate respecting homotopic connectivity. Nat. Commun. 2017, 8, 217. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Whiteway, M.R.; Sheikhattar, A.; Butts, D.A.; Babadi, B.; Kanold, P.O. Parallel Processing of Sound Dynamics across Mouse Auditory Cortex via Spatially Patterned Thalamic Inputs and Distinct Areal Intracortical Circuits. Cell Rep. 2019, 27, 872–885.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dombeck, D.; Tank, D. Two-Photon Imaging of Neural Activity in Awake Mobile Mice. Cold Spring Harb. Protoc. 2014, 2014, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.B.; Diamanti, E.M.; Fournier, J.; Harris, K.D.; Carandini, M. Coherent encoding of subjective spatial position in visual cortex and hippocampus. Nature 2018, 562, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Wekselblatt, J.B.; Flister, E.D.; Piscopo, D.M.; Niell, C.M. Large-scale imaging of cortical dynamics during sensory perception and behavior. J. Neurophysiol. 2016, 115, 2852–2866. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liang, G.; Liu, F.; Chen, Q.; Xi, L. A Long-Term Cranial Window for High-Resolution Photoacoustic Imaging. IEEE Trans. Biomed. Eng. 2021, 68, 706–711. [Google Scholar] [CrossRef]
- Takehara, H.; Nagaoka, A.; Noguchi, J.; Akagi, T.; Kasai, H.; Ichiki, T. Lab-on-a-brain: Implantable micro-optical fluidic devices for neural cell analysis in vivo. Sci. Rep. 2014, 4, 6721. [Google Scholar] [CrossRef] [Green Version]
- Helm, P.J.; Ottersen, O.P.; Nase, G. Analysis of optical properties of the mouse cranium—Implications for in vivo multi photon laser scanning microscopy. J. Neurosci. Methods 2009, 178, 316–322. [Google Scholar] [CrossRef]
- Rosenthal, Z.; Raut, R.V.; Yan, P.; Koko, D.; Kraft, A.W.; Czerniewski, L.; Acland, B.; Mitra, A.; Snyder, L.H.; Bauer, A.Q.; et al. Local Perturbations of Cortical Excitability Propagate Differentially Through Large-Scale Functional Networks. Cereb. Cortex 2020, 30, 3352–3369. [Google Scholar] [CrossRef]
- Gong, X.; Mendoza-Halliday, D.; Ting, J.T.; Kaiser, T.; Sun, X.; Bastos, A.M.; Wimmer, R.D.; Guo, B.; Chen, Q.; Zhou, Y.; et al. An Ultra-Sensitive Step-Function Opsin for Minimally Invasive Optogenetic Stimulation in Mice and Macaques. Neuron 2020, 107, 38–51.e8. [Google Scholar] [CrossRef]
- Miao, P.; Zhang, L.; Li, M.; Zhang, Y.; Feng, S.; Wang, Q.; Thakor, N.V. Chronic wide-field imaging of brain hemodynamics in behaving animals. Biomed. Opt. Express 2016, 8, 436–445. [Google Scholar] [CrossRef] [Green Version]
- Ghanbari, L.; Carter, R.E.; Rynes, M.L.; Dominguez, J.; Chen, G.; Naik, A.; Hu, J.; Sagar, A.K.; Haltom, L.; Mossazghi, N.; et al. Cortex-wide neural interfacing via transparent polymer skulls. Nat. Commun. 2019, 10, 1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.H.; Zhang, Y.; Lecoq, J.; Jung, J.C.; Li, J.; Zeng, H.; Niell, C.M.; Schnitzer, M.J. Long-Term Optical Access to an Estimated One Million Neurons in the Live Mouse Cortex. Cell Rep. 2016, 17, 3385–3394. [Google Scholar] [CrossRef] [PubMed]
- Kunori, N.; Takashima, I. An Implantable Cranial Window Using a Collagen Membrane for Chronic Voltage-Sensitive Dye Imaging. Micromachines 2019, 10, 789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, I.T.; Symon, M.R.; Bristol, R.E.; Beals, S.P.; Joganic, E.F.; Adelson, P.D.; Shafron, D.H.; Singh, D.J. Outcomes of Titanium Mesh Cranioplasty in Pediatric Patients. J. Craniofac. Surg. 2018, 29, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, B.; Xie, Z.; Ding, S.; Zhuang, Z.; Lin, L.; Guo, Y.; Chen, H.; Yu, X. Comparison of manually shaped and computer-shaped titanium mesh for repairing large frontotemporoparietal skull defects after traumatic brain injury. Neurosurg. Focus 2012, 33, E13. [Google Scholar] [CrossRef] [Green Version]
- Lam, S.; Kuether, J.; Fong, A.; Reid, R. Cranioplasty for Large-Sized Calvarial Defects in the Pediatric Population: A Review. Craniomaxillofac. Trauma Reconstr. 2015, 8, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, J.A.; Paliga, J.T.; Bartlett, S.P. Cranioplasty. Curr. Opin. Otolaryngol. Head Neck Surg. 2013, 21, 400–409. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, Y.; Lin, F.; Chen, Z.; Jiang, X.; Zhang, J.; Wang, J. Complications following titanium cranioplasty compared with nontitanium implants cranioplasty: A systematic review and meta-analysis. J. Clin. Neurosci. 2021, 84, 66–74. [Google Scholar] [CrossRef]
- Lewitz, M.; Salma, A.; Saravia, H.W.; Sakellaropoulou, I.; Sarkis, H.M.; Ewelt, C.; Fortmann, T.; Wilbers, E.; Schipmann, S.; Molina, E.S.; et al. Load-Bearing Capacity and Design Advantages of a Custom-Made, Thin Pure-Titanium Cranioplasty (CranioTop). J. Craniofac. Surg. 2021, 32, 1291–1296. [Google Scholar] [CrossRef]
- Maher, S.; Kaur, G.; Lima-Marques, L.; Evdokiou, A.; Losic, D. Engineering of Micro- to Nanostructured 3D-Printed Drug-Releasing Titanium Implants for Enhanced Osseointegration and Localized Delivery of Anticancer Drugs. ACS Appl. Mater. Interfaces 2017, 9, 29562–29570. [Google Scholar] [CrossRef]
- Chen, L.M.; Heider, B.; Williams, G.V.; Healy, F.L.; Ramsden, B.M.; Roe, A.W. A chamber and artificial dura method for long-term optical imaging in the monkey. J. Neurosci. Methods 2002, 113, 41–49. [Google Scholar] [CrossRef]
- Feng, P.; Wang, X.; Lu, B.; Pan, G.; Leng, X.; Ma, X.; Zhang, J.; Zhao, W. Ionic liquids-filled patterned cavities improve transmittance of transparent and stretchable electronic polydimethylsiloxane films. J. Mater. Sci. 2019, 54, 11134–11144. [Google Scholar] [CrossRef]
- Guan, F.; Song, Z.; Xin, F.; Wang, H.; Yu, D.; Li, G.; Liu, W. Preparation of hydrophobic transparent paper via using polydimethylsiloxane as transparent agent. J. Bioresour. Bioprod. 2020, 5, 37–43. [Google Scholar] [CrossRef]
- Świerczek-Lasek, B.; Keremidarska-Markova, M.; Hristova-Panusheva, K.; Vladkova, T.; Ciemerych, M.A.; Archacka, K.; Krasteva, N. Polydimethylsiloxane materials with supraphysiological elasticity enable differentiation of myogenic cells. J. Biomed. Mater. Res. Part A 2019, 107, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Shtoyerman, E.; Arieli, A.; Slovin, H.; Vanzetta, I.; Grinvald, A. Long-Term Optical Imaging and Spectroscopy Reveal Mechanisms Underlying the Intrinsic Signal and Stability of Cortical Maps in V1 of Behaving Monkeys. J. Neurosci. 2000, 20, 8111–8121. [Google Scholar] [CrossRef] [Green Version]
- Ryu, M.; Kim, J.; Lee, S.; Kim, J.; Song, T. Stretchable and Transparent Paper Based on PDMS–CNC Composite for Direct Printing. Adv. Mater. Technol. 2021, 6, 2100156. [Google Scholar] [CrossRef]
- Borók, A.; Laboda, K.; Bonyár, A. PDMS Bonding Technologies for Microfluidic Applications: A Review. Biosensors 2021, 11, 292. [Google Scholar] [CrossRef]
- Martínez-Aviñó, A.; Hakobyan, L.; Ballester-Caudet, A.; Moliner-Martínez, Y.; Molins-Legua, C.; Campíns-Falcó, P. NQS-Doped PDMS Solid Sensor: From Water Matrix to Urine Enzymatic Application. Biosensors 2021, 11, 186. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, W.; Fan, X.; Wang, J.; Fu, S.; Cui, S.; Liao, F.; Cai, J.; Wang, X.; Huang, Y.; et al. Upregulation of interleukin-6 on Cav3.2 T-type calcium channels in dorsal root ganglion neurons contributes to neuropathic pain in rats with spinal nerve ligation. Exp. Neurol. 2019, 317, 226–243. [Google Scholar] [CrossRef]
- Chen, W.; Chi, Y.-N.; Kang, X.-J.; Liu, Q.-Y.; Zhang, H.-L.; Li, Z.-H.; Zhao, Z.-F.; Yang, Y.; Su, L.; Cai, J.; et al. Accumulation of Cav3.2 T-type Calcium Channels in the Uninjured Sural Nerve Contributes to Neuropathic Pain in Rats with Spared Nerve Injury. Front. Mol. Neurosci. 2018, 11, 24. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.-Y.; Chen, W.; Cui, S.; Liao, F.-F.; Yi, M.; Liu, F.-Y.; Wan, Y. Upregulation of Cav3.2 T-type calcium channels in adjacent intact L4 dorsal root ganglion neurons in neuropathic pain rats with L5 spinal nerve ligation. Neurosci. Res. 2019, 142, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, F.-Y.; Tong, Z.-Q.; Li, Z.-H.; Chen, W.; Luo, W.-H.; Li, H.; Luo, H.-J.; Tang, Y.; Tang, J.-M.; et al. Lysine-Specific Demethylase 1 in Breast Cancer Cells Contributes to the Production of Endogenous Formaldehyde in the Metastatic Bone Cancer Pain Model of Rats. PLoS ONE 2013, 8, e58957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.-Y.; Sun, Y.-N.; Wang, F.-T.; Li, Q.; Su, L.; Zhao, Z.-F.; Meng, X.-L.; Zhao, H.; Wu, X.; Sun, Q.; et al. Activation of satellite glial cells in lumbar dorsal root ganglia contributes to neuropathic pain after spinal nerve ligation. Brain Res. 2012, 1427, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-Y.; Qu, X.-X.; Ding, X.; Cai, J.; Jiang, H.; Wan, Y.; Han, J.-S.; Xing, G.-G. Decrease in the descending inhibitory 5-HT system in rats with spinal nerve ligation. Brain Res. 2010, 1330, 45–60. [Google Scholar] [CrossRef]
- Sun, H.; Fu, S.; Cui, S.; Yin, X.; Sun, X.; Qi, X.; Cui, K.; Wang, J.; Ma, L.; Liu, F.-Y.; et al. Development of a CRISPR-SaCas9 system for projection- and function-specific gene editing in the rat brain. Sci. Adv. 2020, 6, eaay6687. [Google Scholar] [CrossRef] [Green Version]
- Kratskin, I.L.; Yu, X.; Doty, R.L. An Easily Constructed Pipette for Pressure Microinjections into the Brain. Brain Res. Bull. 1997, 44, 199–203. [Google Scholar] [CrossRef]
- Jiang, Y.-Y.; Shao, S.; Zhang, Y.; Zheng, J.; Chen, X.; Cui, S.; Liu, F.-Y.; Wan, Y.; Yi, M. Neural pathways in medial septal cholinergic modulation of chronic pain: Distinct contribution of the anterior cingulate cortex and ventral hippocampus. Pain 2018, 159, 1550–1561. [Google Scholar] [CrossRef]
- Zheng, J.; Jiang, Y.-Y.; Xu, L.-C.; Ma, L.-Y.; Liu, F.-Y.; Cui, S.; Cai, J.; Liao, F.-F.; Wan, Y.; Yi, M. Adult Hippocampal Neurogenesis along the Dorsoventral Axis Contributes Differentially to Environmental Enrichment Combined with Voluntary Exercise in Alleviating Chronic Inflammatory Pain in Mice. J. Neurosci. 2017, 37, 4145–4157. [Google Scholar] [CrossRef]
- Wang, Y.; Shah, P.; Phillips, C.; Sims, C.E.; Allbritton, N.L. Trapping cells on a stretchable microwell array for single-cell analysis. Anal. Bioanal. Chem. 2011, 402, 1065–1072. [Google Scholar] [CrossRef] [Green Version]
- Torimitsu, S.; Nishida, Y.; Takano, T.; Yajima, D.; Inokuchi, G.; Makino, Y.; Motomura, A.; Chiba, F.; Yamaguchi, R.; Hashimoto, M.; et al. Differences in biomechanical properties and thickness among frontal and parietal bones in a Japanese sample. Forensic Sci. Int. 2015, 252, 190.e1–190.e6. [Google Scholar] [CrossRef]
- Pandey, A.; Gupta, P.; Vairagi, K.; Mondal, S.K. Packaged Negative Axicon Optical Fiber Probe and Bessel Beam Interferometry for Refractive Index Measurement of Hazardous Liquid Samples. J. Light. Technol. 2019, 37, 6121–6126. [Google Scholar] [CrossRef]
- Bolin, F.P.; Preuss, L.E.; Taylor, R.C.; Ference, R.J. Refractive index of some mammalian tissues using a fiber optic cladding method. Appl. Opt. 1989, 28, 2297–2303. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, N.; Liu, F.; Zhang, X.; Chen, C.; Xia, Z.; Fu, S.; Wang, J.; Xu, J.; Cui, S.; Zhang, Y.; et al. A Hybrid Titanium-Softmaterial, High-Strength, Transparent Cranial Window for Transcranial Injection and Neuroimaging. Biosensors 2022, 12, 129. https://doi.org/10.3390/bios12020129
Yang N, Liu F, Zhang X, Chen C, Xia Z, Fu S, Wang J, Xu J, Cui S, Zhang Y, et al. A Hybrid Titanium-Softmaterial, High-Strength, Transparent Cranial Window for Transcranial Injection and Neuroimaging. Biosensors. 2022; 12(2):129. https://doi.org/10.3390/bios12020129
Chicago/Turabian StyleYang, Nana, Fengyu Liu, Xinyue Zhang, Chenni Chen, Zhiyuan Xia, Su Fu, Jiaxin Wang, Jingjing Xu, Shuang Cui, Yong Zhang, and et al. 2022. "A Hybrid Titanium-Softmaterial, High-Strength, Transparent Cranial Window for Transcranial Injection and Neuroimaging" Biosensors 12, no. 2: 129. https://doi.org/10.3390/bios12020129
APA StyleYang, N., Liu, F., Zhang, X., Chen, C., Xia, Z., Fu, S., Wang, J., Xu, J., Cui, S., Zhang, Y., Yi, M., Wan, Y., Li, Q., & Xu, S. (2022). A Hybrid Titanium-Softmaterial, High-Strength, Transparent Cranial Window for Transcranial Injection and Neuroimaging. Biosensors, 12(2), 129. https://doi.org/10.3390/bios12020129