Gold Nanoparticles Prepared with Cyclodextrin Applied to Rapid Vertical Flow Technology for the Detection of Brucellosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Reagents
2.2. Laboratory Apparatus
2.3. Preparation of Gold Nanoparticles
2.4. Marker Protein A
2.5. Preparation of Silver Dye Reinforcing Solution
2.6. Assembly of Rapid Vertical Flow Technology Device
2.7. Selection of NC Film
2.8. Optimization of Coating Antigen
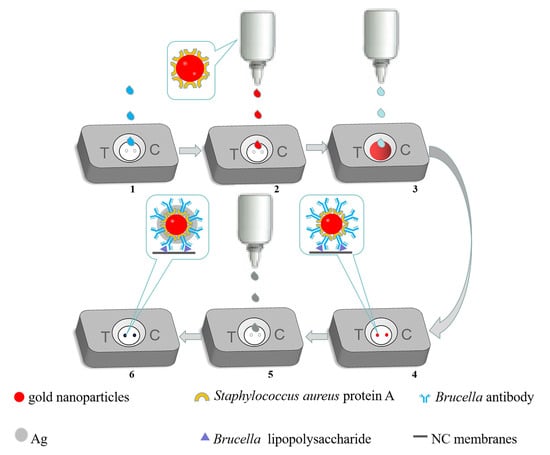
2.9. Implementation of the Test Steps and Interpretation of the Test Results
3. Results
3.1. Optimization of the Synthesis Process of Gold Nanoparticles
3.2. Characterization of Gold Nanoparticles
3.3. Optimization of Marker SPA
3.4. Optimization of Synthetic Buffer
3.5. Detection of the Specificity of the Diafiltration Card
3.6. Detection of the Detection Limit of the Percolating Card
3.7. Determination of Optimal Silver Staining Incubation Time
3.8. Silver Staining Results
3.9. Repeatability and Stability Evaluation of RVFT
3.10. The Advantages of RVFT Compared with Lateral Flow Immunoassay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, S.B.; Xiao, D.; Liu, J.Y.; Bi, H.M.; Zheng, Z.R.; Wang, L.D.; Yang, X.W.; Tian, G.Z.; Zhao, H.Y.; Piao, D.R.; et al. Fluorescence polarization assay improves the rapid detection of human brucellosis in China. Infect. Dis. Poverty 2021, 10, 46. [Google Scholar] [CrossRef]
- Betsy, J.B. PCR as a diagnostic tool for brucellosis. Vet. Microbiol. 2002, 90, 435–446. [Google Scholar]
- Sumi, S. The Demographic Risk Factors for Brucellosis in Asia. Asian J. Res. Infect. Dis. 2021, 7, 6–13. [Google Scholar]
- Marianelli, C.; Ciuchini, F.; Tarantino, M.; Pasquali, P.; Adone, R. Molecular characterization of the rpoB gene in Brucella species: New potential molecular markers for genotyping. Microbes Infect. 2006, 8, 860–865. [Google Scholar] [CrossRef]
- Scholz, H.C.; Tomaso, H.; Al, D.S.; Witte, A.; Schloter, M.; Kämpfer, P.; Falsen, E.; Neubauer, H. Genotyping of Ochrobactrum anthropi by recA-based comparative sequence, PCR-RFLP, and 16S rRNA gene analysis. FEMS Microbiol. Lett. 2006, 257, 7–16. [Google Scholar] [CrossRef] [Green Version]
- López-Goñi, I.; García-Yoldi, D.; Marín, C.M.; Miguel, M.J.; Barquero-Calvo, E.; Guzmán-Verri, C.; Albert, D.; Garin-Bastuji, B. New Bruce-ladder multiplex PCR assay for the biovar typing of Brucella suis and the discrimination of Brucella suis and Brucella canis. Vet. Microbiol. 2011, 154, 152–155. [Google Scholar] [CrossRef]
- Navarro, E.; Casao, M.A.; Solera, J. Diagnosis of human brucellosis using PCR. Expert Rev. Mol. Diagn. 2004, 4, 115. [Google Scholar] [CrossRef]
- Al-Attas, R.A.; Al-Khalifa, M.; Al-Qurashi, A.R.; Badawy, M.; Al-Gualy, N. Evaluation of PCR, culture and serology for the diagnosis of acute human brucellosis. Ann. Saudi Med. 2000, 20, 224–228. [Google Scholar] [CrossRef]
- Sascha, A.D.; Nöckler, K. Implications of laboratory diagnosis on brucellosis therapy. Expert Rev. Anti-Infect. Ther. 2011, 9, 833–845. [Google Scholar]
- Sascha, A.D.; Herbert, T.; Karsten, N.; Heinrich, N.; Dimitrios, F. Laboratory-based diagnosis of brucellosis—A review of the literature. Part II: Serological tests for brucellosis. Clin. Lab. 2003, 49, 577–589. [Google Scholar]
- Matar, G.M.; Khneisser, I.A.; Abdelnoor, A.M. Rapid laboratory confirmation of human brucellosis by PCR analysis of a target sequence on the 31-kilodalton Brucella antigen DNA. J. Clin. Microbiol. 1996, 34, 477–478. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Lago, L.; Vallejo, F.J.; Trujillano, I.; Vizcaíno, N. Fluorescent Whole-Cell Hybridization with 16S rRNA-Targeted Oligonucleotide Probes To Identify Brucella spp. by Flow Cytometry. J. Clin. Microbiol. 2000, 38, 2768–2771. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Wang, Y.; Liu, Y.; Huang, J.; Tan, Q.; Ying, X.; Hu, Y.; Li, S. A Label-Based Polymer Nanoparticles Biosensor Combined with Loop-Mediated Isothermal Amplification for Rapid, Sensitive, and Highly Specific Identification of Brucella abortus. Front. Bioeng. Biotechnol. 2021, 9, 758564. [Google Scholar] [CrossRef]
- Mishra, A.; Thomas, P.; Jeena, L.M.; Doimari, S.; Rajagunalan, S.; Sahoo, A.P.; Singh, D.K.; Al, D.S.; Sprague, L.D.; Neubauer, H.; et al. A novel close-tube loop mediated isothermal amplification (Lamp) assay for rapid detection of Brucella. Indian J. Anim. Res. 2021, 1, 8. [Google Scholar] [CrossRef]
- Lin, G.Z. Loop-mediated isothermal amplification assay targeting the omp25 gene for rapid detection of Brucella spp. Mol. Cell. Probes 2011, 25, 126–129. [Google Scholar] [CrossRef]
- Gad, E.M.O.; Kambal, A.M. Evaluation of a Brucella enzyme immunoassay test (ELISA) in comparison with bacteriological culture and agglutination. J. Infect. 1998, 36, 197–201. [Google Scholar]
- Kuyumcu, C.A.; Erol, S.; Adaleti, R.; Senbayrak, S.; Barkay, O. Comparison of Coombs Gel Test with ELISA and Standard Tube Agglutination Tests Used in Serological Diagnosis of Brucellosis. Infect. Dis. Clin. Microbiol. 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Memish, Z.A.; Almuneef, M.; Mah, M.W.; Qassem, L.A.; Osoba, A.O. Comparison of the Brucella Standard Agglutination Test with the ELISA IgG and IgM in patients with Brucella bacteremia. Diagn. Microbiol. Infect. Dis. 2002, 44, 129–132. [Google Scholar] [CrossRef]
- Corbel, M.J. The relationship between the protective and cross-reacting antigens of Brucella spp., Yersinia enterocolitica 0:9 and Salmonella serotypes of Kauffmann-White group N. Contrib. Microbiol. Immunol. 1979, 5, 50. [Google Scholar]
- Corbel, M.J. Recent advances in the study of Brucella antigens and their serological cross-reactions. Vet. Bull. 1985, 55, 927–942. [Google Scholar]
- Giovanni, D.B.; Silvia, A.; Andrea, I.; Tommasangelo, P.; Giovanni, G. Microbiological Laboratory Diagnosis of Human Brucellosis: An Overview. Pathogens 2021, 10, 1623. [Google Scholar]
- Gomez, M.C.; Nieto, J.A.; Rosa, C.; Geijo, P.; Escribano, M.A.; Munoz, A.; Lopez, C. Evaluation of seven tests for the diagnosis of human brucellosis in an endemic area. Clin. Vaccine Immunol. 2008, 15, 1031–1033. [Google Scholar] [CrossRef] [Green Version]
- Blasco, J.M.; Garin-Bastuji, B.; Marin, C.M.; Gerbier, G.; Fanlo, J.; Jimenez de Bagues, M.P.; Cau, C. Efficacy of different rose bengal and complement fixation antigens for the diagnosis of Brucella melitensis in sheep and goats. Vet. Rec. 1994, 134, 415–420. [Google Scholar] [CrossRef]
- Blasco, J.M.; Mar ın, C.; Jimenez de Bagues, M.P.; Barberán, M.; Hernández, A.; Molina, L.; Velasco, J.D.; Díaz, R.; Moriyón, I. Evaluation of allergic and serological tests for diagnosing Brucella melitensis infection in sheep. J. Clin. Microbiol. 1994, 32, 1835–1840. [Google Scholar] [CrossRef] [Green Version]
- Spielberg, F.; Ryder, R.; Harris, J.; Heyward, W.; Kabeya, C.; Kifuani, N.K.; Bender, T.; Quinn, T. Field testing and comparative evaluation of rapid, visually read screening assays for antibody to human immunodeficiency virus. Lancet 1989, 333, 580–584. [Google Scholar] [CrossRef]
- Debjani, S.; Dipika, R.; Tarun, K.D. Immunofiltration assay for aflatoxin B 1 based on the separation of pre-immune complexes. J. Immunol. Methods 2013, 392, 24–28. [Google Scholar]
- Zhang, P.; Bao, Y.; Draz, M.S.; Lu, H.; Liu, C.; Han, H. Rapid and quantitative detection of C-reactive protein based on quantum dots and immunofiltration assay. Int. J. Nanomed. 2015, 10, 6161. [Google Scholar] [CrossRef] [Green Version]
- Burmistrova, N.A.; Rusanova, T.Y.; Yurasov, N.A.; Saege, S.R.; Goryacheva, I.Y. Simultaneous Determination of Several Mycotoxins by Rapid Immunofiltration Assay. J. Anal. Chem. 2014, 69, 525–534. [Google Scholar] [CrossRef]
- Schleh, C.; Semmler-Behnke, M.; Lipka, J.; Wenk, A.; Hirn, S.; Schäffler, M.; Schmid, G.; Simon, U.; Kreyling, W.G. Size and surface charge of gold nanoparticles determine absorption across intestinal barriers and accumulation in secondary target organs after oral administration. Nanotoxicology 2012, 6, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Morin-Crini, N.; Fourmentin, S.; Éva, F.; Lichtfouse, E.; Torri, G.; Fourmentin, M.; Crini, G. 130 years of cyclodextrin discovery for health, food, agriculture, and the industry: A review. Environ. Chem. Lett. 2021, 19, 2581–2617. [Google Scholar] [CrossRef]
- Petitjean, M.; García-Zubiri, I.X.; Isasi, J.R. History of cyclodextrin-based polymers in food and pharmacy: A review. Environ. Chem. Lett. 2021, 19, 3465–3476. [Google Scholar] [PubMed]
- Connors, K.A. ChemInform Abstract: The Stability of Cyclodextrin Complexes in Solution. ChemInform 1997, 28. [Google Scholar] [CrossRef]
- Lysik, M.A.; Wu-Pong, S. Innovations in Oligonucleotide Drug Delivery. J. Pharm. Sci. 2003, 92, 1559–1573. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. ChemInform 2003, 107, 668–677. [Google Scholar]
- Bakar, F.; Caglayan, M.G.; Onur, F.; Nebioglu, S.; Palabiyik, I.M. Gold nanoparticle-lignan complexes inhibited MCF-7 cell proliferation in vitro: A novel conjugation for cancer therapy. Anti-Cancer Agents Med. Chem. 2015, 15, 344–364. [Google Scholar] [CrossRef]
- Zhongliang, D.; Yuetao, G.; Qike, C.; Ke, H. The detection of a transgenic soybean biochip using gold label silver stain technology. Bioorg. Med. Chem. Lett. 2011, 21, 6905–6908. [Google Scholar]
- Ducrotoy, M.J.; Conde-Álvarez, R.; Blasco, J.M.; Moriyón, I. A review of the basis of the immunological diagnosis of ruminant brucellosis. Vet. Immunol. Immunopathol. 2016, 171, 81–102. [Google Scholar] [CrossRef]
- Zuber, A.; Purdey, M.; Schartner, E.; Forbes, C.; Hoek, B.; Giles, D.; Abell, A.; Monro, T.; Heike, E. Detection of gold nanoparticles with different sizes using absorption and fluorescence based method. Sens. Actuators B Chem. 2016, 227, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Zeng, S.; Xia, Y.; Law, W.C.; Zhang, Y.; Rui, H.; Dinh, X.Q.; Ho, H.P.; Yong, K.T. Size dependence of Au NP-enhanced surface plasmon resonance based on differential phase measurement. Sens. Actuators B Chem. 2013, 176, 1128–1133. [Google Scholar] [CrossRef]
- Kumari, Y.; Kaur, G.; Kumar, R.; Singh, S.K.; Gulati, M.; Khursheed, R.; Clarisse, A.; Gowthamarajan, K.; Karri, V.N.R.; Mahalingam, R.; et al. Gold nanoparticles: New routes across old boundaries. Adv. Colloid. Interfac. 2019, 274, 102037. [Google Scholar] [CrossRef]
- Essis Yei, L.H.; Beden, B.; Lamy, C. Electrocatalytic oxidation of glucose at platinum in alkaline medium: On the role of temperature. J. Electroanal. Chem. Interfacial Electrochem. 1988, 246, 349–362. [Google Scholar]
- Pamies, R.; Zhu, K.; Kjøniksen, A.; Nyström, B. Temperature effects on the stability of gold nanoparticles in the presence of a cationic thermoresponsive copolymer. J. Nanoparticle Res. Interdiscip. Forum Nanoscale Sci. Technol. 2016, 18, 319. [Google Scholar] [CrossRef]
- Jia, M.; Liu, J.; Zhang, J.; Zhang, H. An immunofiltration strip method based on the photothermal effect of gold nanoparticles for the detection of Escherichia coli O157:H7. Analyst 2019, 144, 573–578. [Google Scholar] [CrossRef]
- Chandan, P.; Bablu, K.; Prasad, S.R.; Praveen, S.; Garima, S.; Aparajita, D.; Marcia, A.; Abhishek; Pal, S.K.; Kumar, S.M.; et al. Development and evaluation of a gold nanoparticle based Lateral Flow assay (LFA) strip test for detection of Brucella spp. J. Microbiol. Methods 2021, 184, 106185. [Google Scholar]
- Fernando, S.A.; Wilson, G.S. Studies of the ‘hook’ effect in the one-step sandwich immunoassay. J. Immunol. Methods 1992, 151, 47–66. [Google Scholar] [CrossRef]















| Standard Brucellosis Positive Serum | Test Card Box | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | I | II | III | ||
| Test card box | + | + | + | standard brucellosis positive serum | + | + | + |
| Test card box | + | + | + | standard brucellosis positive serum | + | + | + |
| Test card box | + | + | + | standard brucellosis positive serum | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, A.; Feng, D.; Luo, X.; Shi, F. Gold Nanoparticles Prepared with Cyclodextrin Applied to Rapid Vertical Flow Technology for the Detection of Brucellosis. Biosensors 2022, 12, 531. https://doi.org/10.3390/bios12070531
Fang A, Feng D, Luo X, Shi F. Gold Nanoparticles Prepared with Cyclodextrin Applied to Rapid Vertical Flow Technology for the Detection of Brucellosis. Biosensors. 2022; 12(7):531. https://doi.org/10.3390/bios12070531
Chicago/Turabian StyleFang, Ashe, Danni Feng, Xiushuang Luo, and Feng Shi. 2022. "Gold Nanoparticles Prepared with Cyclodextrin Applied to Rapid Vertical Flow Technology for the Detection of Brucellosis" Biosensors 12, no. 7: 531. https://doi.org/10.3390/bios12070531
APA StyleFang, A., Feng, D., Luo, X., & Shi, F. (2022). Gold Nanoparticles Prepared with Cyclodextrin Applied to Rapid Vertical Flow Technology for the Detection of Brucellosis. Biosensors, 12(7), 531. https://doi.org/10.3390/bios12070531