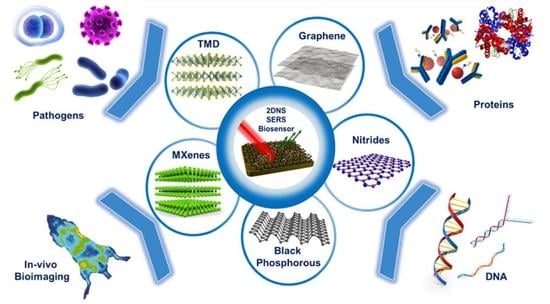
Engineered Two-Dimensional Nanostructures as SERS Substrates for Biomolecule Sensing: A Review
Abstract
:1. Introduction
2. 2DNS as SERS Substrates
2.1. Graphene SERS (GERS) Substrates
2.2. Nitrides SERS Substrates
2.3. Black Phosphorous (BP) SERS Substrates
2.4. MXenes SERS Substrates
2.5. Transition Metal Dichalcogenide (TMD) SERS Substrates
2.6. Metal Oxide SERS Substrates
2.7. 2D MOFs/COFs SERS Substrates
3. 2DNS-Based SERS Biomolecule Sensors
4. 2DNS as Support for Plasmonic Nanostructure in SERS Biosensors
4.1. Graphene-Supported SERS Substrates
4.2. Nitrides-Supported SERS Substrates
4.3. Black Phosphorous-Supported SERS Substrates
4.4. MXene-Supported SERS Substrates
4.5. Transition Metal Dichalcogenide-Supported SERS Substrates
4.6. 2D MOF/COF-Supported SERS Substrates
5. A Comparative Statement
6. Current Technological Challenges and Opportunities
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2DNS | Two-dimensional nanostructures |
| AgNPs | Silver Nanoparticles |
| AlN | Aluminium Nitride |
| AuNPs | Gold Nanoparticles |
| BP | Black Phosphorus |
| CFU | Colony-Forming unit |
| CM | Chemical Mechanism |
| COFs | Covalent Organic Frameworks |
| CT | Charge Transfer |
| CV | Crystal Violet |
| CVD | Chemical Vapour Deposition |
| DNA | Deoxyribonucleic acid |
| ECL | Electrochemiluminescence |
| EF | Enhancement Factor |
| EM | Electromagnetic Mechanism |
| g-C3N4 | graphitic carbon nitride |
| GERS | Graphene Enhanced Raman Scattering |
| GO | Graphene Oxide |
| h-BN | hexagonal Boron Nitride |
| HIV | Human immunodeficiency virus |
| HOMO | Highest Occupied Molecular Orbital |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| LSPR | Localized Surface Plasmon Resonance |
| LUMO | Lowest unoccupied Molecular Orbital |
| MB | Methylene Blue |
| MG | Malachite Green |
| MOFs | Metal Oxide Frameworks |
| MoOx | Molybdenum Oxide |
| MoS2 | Molybdenum Sulphate |
| NPs | Nanoparticles |
| NWs | Nanowires |
| OTA | Ochratoxin |
| PCA | Principal Component Analysis |
| rGO | reduced Graphene Oxide |
| Rh6G | Rhodamine 6G |
| RhB | Rhodamine B |
| SERS | Surface-Enhanced Raman Scattering |
| TaN | Tantalum Nitride |
| TiAlN | Titanium Aluminium Nitride |
| TiN | Titanium Nitride |
| TiO2 | Titanium Oxide |
| TMDs | Transition-metal dichalcogenides |
| WN | Tungsten Nitride |
| WOx | Tungsten Oxide |
| WS2 | Tungsten Sulphate |
References
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Arlett, J.L.; Myers, E.B.; Roukes, M.L. Comparative advantages of mechanical biosensors. Nat. Nanotechnol. 2011, 6, 203–215. [Google Scholar] [CrossRef] [Green Version]
- Murugasenapathi, N.K.; Ghosh, R.; Ramanathan, S.; Ghosh, S.; Chinnappan, A.; Mohamed, S.A.J.; Esther Jebakumari, K.A.; Gopinath, S.C.B.; Ramakrishna, S.; Palanisamy, T. Transistor-Based Biomolecule Sensors: Recent Technological Advancements and Future Prospects. Crit. Rev. Anal. Chem. 2021, 1–22. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single molecule detection using surface-enhanced Raman scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667. [Google Scholar] [CrossRef] [Green Version]
- Jeanmaire, D.L.; Van Duyne, R.P. Surface raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. Interfacial Electrochem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Creighton, J.A. Anomalously intense Raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar] [CrossRef]
- Moskovits, M. Surface roughness and the enhanced intensity of Raman scattering by molecules adsorbed on metals. J. Chem. Phys. 1978, 69, 4159–4161. [Google Scholar] [CrossRef]
- Managò, S.; Quero, G.; Zito, G.; Tullii, G.; Galeotti, F.; Pisco, M.; De Luca, A.C.; Cusano, A. Tailoring lab-on-fiber SERS optrodes towards biological targets of different sizes. Sens. Actuators B Chem. 2021, 339, 129321. [Google Scholar] [CrossRef]
- Fang, X.; Zheng, C.; Yin, Z.; Wang, Z.; Wang, J.; Liu, J.; Luo, D.; Liu, Y.J. Hierarchically Ordered Silicon Metastructures from Improved Self-Assembly-Based Nanosphere Lithography. ACS Appl. Mater. Interfaces 2020, 12, 12345–12352. [Google Scholar] [CrossRef]
- Pisco, M.; Galeotti, F.; Grisci, G.; Quero, G.; Cusano, A. Self-assembled periodic patterns on the optical fiber tip by microsphere arrays. In Proceedings of the 24th International Conference on Optical Fibre Sensors, Curitiba, Brazil, 28 September–2 October 2015; pp. 165–168. [Google Scholar]
- Gersten, J.I.; Birke, R.L.; Lombardi, J.R. Theory of enhance i light scattering from molecules adsorbed at the metal-solution interface. Phys. Rev. Lett. 1979, 43, 147. [Google Scholar] [CrossRef]
- Ru, E.L.; Etchegoin, P. Principles of Surface-Enhanced Raman Spectroscopy: And Related Plasmonic Effects; Elsevier Science: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kadam, U.S.; Craig, A.P.; Irudayaraj, J. In Vivo Biodetection using Surface-Enhanced Raman Spectroscopy; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2014. [Google Scholar]
- Kadam, U.S.; Chavhan, R.L.; Schulz, B.; Irudayaraj, J. Single molecule Raman spectroscopic assay to detect transgene from GM plants. Anal. Biochem. 2017, 532, 60–63. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Murali, A.; Lokhande, G.; Deo, K.A.; Brokesh, A.; Gaharwar, A.K. Emerging 2D nanomaterials for biomedical applications. Mater. Today 2021, 50, 276–302. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, X.; Gong, F.; Liu, T.; Liu, Z. 2D Nanomaterials for Cancer Theranostic Applications. Adv. Mater. 2020, 32, 1902333. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Zhao, M.; Liu, G.; Wu, J. 2D nanomaterials for tissue engineering application. Nano Res. 2020, 13, 2019–2034. [Google Scholar] [CrossRef]
- Karthick Kannan, P.; Shankar, P.; Blackman, C.; Chung, C.H. Recent Advances in 2D Inorganic Nanomaterials for SERS Sensing. Adv. Mater. 2019, 31, 1803432. [Google Scholar] [CrossRef]
- Serafinelli, C.; Fantoni, A.; Alegria, E.C.B.A.; Vieira, M. Plasmonic Metal Nanoparticles Hybridized with 2D Nanomaterials for SERS Detection: A Review. Biosensors 2022, 12, 225. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Zhou, Y.; Feng, E.; Tian, Y. Surface-enhanced Raman Scattering on 2D Nanomaterials: Recent Developments and Applications. Chin. J. Chem. 2021, 39, 745–756. [Google Scholar] [CrossRef]
- Guselnikova, O.; Lim, H.; Kim, H.-J.; Kim, S.H.; Gorbunova, A.; Eguchi, M.; Postnikov, P.; Nakanishi, T.; Asahi, T.; Na, J.; et al. New Trends in Nanoarchitectured SERS Substrates: Nanospaces, 2D Materials, and Organic Heterostructures. Small 2022, 18, 2107182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Xing, Z.; Wan, D.; Gao, H.; Han, Y.; Gao, Y.; Hu, H.; Cheng, Z.; Liu, T. Surface-enhanced Raman spectroscopy chips based on two-dimensional materials beyond graphene. J. Semicond. 2021, 42, 051001. [Google Scholar] [CrossRef]
- Yin, Z.; Xu, K.; Jiang, S.; Luo, D.; Chen, R.; Xu, C.; Shum, P.; Liu, Y.J. Recent progress on two-dimensional layered materials for surface enhanced Raman spectroscopy and their applications. Mater. Today Phys. 2021, 18, 100378. [Google Scholar] [CrossRef]
- Xia, M. A Review on Applications of Two-Dimensional Materials in Surface-Enhanced Raman Spectroscopy. Int. J. Spectrosc. 2018, 2018, 4861472. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Liu, D.; Du, X.; Lo, K.H.; Wang, S.; Zhou, B.; Pan, H. 2D materials: Excellent substrates for surface-enhanced Raman scattering (SERS) in chemical sensing and biosensing. TrAC Trends Anal. Chem. 2020, 130, 115983. [Google Scholar] [CrossRef]
- Nurrohman, D.T.; Chiu, N.F. A Review of Graphene-Based Surface Plasmon Resonance and Surface-Enhanced Raman Scattering Biosensors: Current Status and Future Prospects. Nanomaterials 2021, 11, 216. [Google Scholar] [CrossRef]
- Deng, S.; Xu, W.; Wang, J.; Ling, X.; Wu, J.; Xie, L.; Kong, J.; Dresselhaus, M.S.; Zhang, J. Direct measurement of the Raman enhancement factor of rhodamine 6G on graphene under resonant excitation. Nano Res. 2014, 7, 1271–1279. [Google Scholar] [CrossRef] [Green Version]
- Huh, S.; Park, J.; Kim, Y.S.; Kim, K.S.; Hong, B.H.; Nam, J.M. UV/Ozone-Oxidized Large-Scale Graphene Platform with Large Chemical Enhancement in Surface-Enhanced Raman Scattering. ACS Nano 2011, 5, 9799–9806. [Google Scholar] [CrossRef]
- Yu, X.; Cai, H.; Zhang, W.; Li, X.; Pan, N.; Luo, Y.; Wang, X.; Hou, J.G. Tuning Chemical Enhancement of SERS by Controlling the Chemical Reduction of Graphene Oxide Nanosheets. ACS Nano 2011, 5, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, C.; Bramhaiah, K.; John, N.S.; Ramachandran, B.E. Low cost, ultra-thin films of reduced graphene oxide–Ag nanoparticle hybrids as SERS based excellent dye sensors. Chem. Phys. Lett. 2015, 629, 81–86. [Google Scholar] [CrossRef]
- Shi, G.; Wang, M.; Zhu, Y.; Shen, L.; Wang, Y.; Ma, W.; Chen, Y.; Li, R. A flexible and stable surface-enhanced Raman scattering (SERS) substrate based on Au nanoparticles/Graphene oxide/Cicada wing array. Opt. Commun. 2018, 412, 28–36. [Google Scholar] [CrossRef]
- Yan, Z.-X.; Zhang, Y.L.; Wang, W.; Fu, X.Y.; Jiang, H.-B.; Liu, Y.Q.; Verma, P.; Kawata, S.; Sun, H.B. Superhydrophobic SERS substrates based on silver-coated reduced graphene oxide gratings prepared by two-beam laser interference. ACS Appl. Mater. Interfaces 2015, 7, 27059–27065. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.L.; Zhen, S.J.; Huang, C.Z. One-pot green synthesis of graphene oxide/gold nanocomposites as SERS substrates for malachite green detection. Analyst 2013, 138, 3075–3081. [Google Scholar] [CrossRef]
- Ayhan, M.E. CVD graphene-based flexible and transparent SERS substrate towards L-tyrosine detection. Microelectron. Eng. 2021, 241, 111546. [Google Scholar] [CrossRef]
- Weng, C.; Luo, Y.; Wang, B.; Shi, J.; Gao, L.; Cao, Z.; Duan, G.J. Layer-dependent SERS enhancement of TiS2 prepared by simple electrochemical intercalation. J. Mater. Chem. C 2020, 8, 14138–14145. [Google Scholar] [CrossRef]
- Tao, L.; Chen, K.; Chen, Z.; Cong, C.; Qiu, C.; Chen, J.; Wang, X.; Chen, H.; Yu, T.; Xie, W.; et al. 1T′ Transition Metal Telluride Atomic Layers for Plasmon-Free SERS at Femtomolar Levels. J. Am. Chem. Soc. 2018, 140, 8696–8704. [Google Scholar] [CrossRef]
- Ekoya, B.G.; Shan, Y.; Cai, Y.; Okombi, N.I.; Yue, X.; Xu, M.; Cong, C.; Hu, L.; Qiu, Z.-J.; Liu, R. 2H Tantalum Disulfide Nanosheets as Substrates for Ultrasensitive SERS-Based Sensing. ACS Appl. Nano Mater. 2022, 5, 8913–8920. [Google Scholar] [CrossRef]
- Zheng, Z.; Cong, S.; Gong, W.; Xuan, J.; Li, G.; Lu, W.; Geng, F.; Zhao, Z. Semiconductor SERS enhancement enabled by oxygen incorporation. Nat. Commun. 2017, 8, 1993. [Google Scholar] [CrossRef]
- Fraser, J.P.; Postnikov, P.; Miliutina, E.; Kolska, Z.; Valiev, R.; Švorčík, V.; Lyutakov, O.; Ganin, A.Y.; Guselnikova, O. Application of a 2D Molybdenum Telluride in SERS Detection of Biorelevant Molecules. ACS Appl. Mater. Interfaces 2020, 12, 47774–47783. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, H.; Guo, Y.; Wang, K.; Zhang, Y.; Lan, P.; Guo, J.; Zhang, W.; Zhong, H.; Guo, Z.; et al. Lamellar hafnium ditelluride as an ultrasensitive surface-enhanced Raman scattering platform for label-free detection of uric acid. Photonics Res. 2021, 9, 1039–1047. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Ye, B.; Zhuang, Z.; Lan, P.; Zhang, Y.; Zhong, H.; Liu, H.; Guo, Z.; Liu, Z. Rapid label-free SERS detection of foodborne pathogenic bacteria based on hafnium ditelluride-Au nanocomposites. J. Innov. Opt. Heal. Sci. 2020, 13, 2041004. [Google Scholar] [CrossRef]
- Yuan, H.; Yu, S.; Kim, M.; Lee, J.-E.; Kang, H.; Jang, D.; Ramasamy, M.S.; Kim, D.H. Dopamine-mediated self-assembled anisotropic Au nanoworms conjugated with MoS2 nanosheets for SERS-based sensing. Sens. Actuators B Chem. 2022, 371, 132453. [Google Scholar] [CrossRef]
- Guo, J.; Ding, C.; Gan, W.; Chen, P.; Zhang, M.; Sun, Z. Fabrication of black phosphorous quantum dots and Ag nanoparticles co-sensitized TiO2 nanorod arrays as powerful SERS substrate. J. Alloy. Compd. 2022, 918, 165621. [Google Scholar] [CrossRef]
- Kundu, A.; Rani, R.; Hazra, K.S. Controlled nanofabrication of metal-free SERS substrate on few layered black phosphorus by low power focused laser irradiation. Nanoscale 2019, 11, 16245–16252. [Google Scholar] [CrossRef]
- Lin, C.; Liang, S.; Peng, Y.; Long, L.; Li, Y.; Huang, Z.; Long, N.V.; Luo, X.; Liu, J.; Li, Z.; et al. Visualized SERS Imaging of Single Molecule by Ag/Black Phosphorus Nanosheets. Nanomicro Lett. 2022, 14, 75. [Google Scholar] [CrossRef]
- Kundu, A.; Rani, R.; Ahmad, A.; Kumar, A.; Raturi, M.; Gupta, T.; Khan, R.; Hazra, K.S. Ultrasensitive and label-free detection of prognostic and diagnostic biomarkers of sepsis on a AgNP-laden black phosphorous-based SERS platform. Sensors Diagn. 2022, 1, 449–459. [Google Scholar] [CrossRef]
- Basu, N.; Satya Bharathi, M.S.; Sharma, M.; Yadav, K.; Parmar, A.S.; Soma, V.R.; Lahiri, J. Large Area Few-Layer Hexagonal Boron Nitride as a Raman Enhancement Material. Nanomaterials 2021, 11, 622. [Google Scholar] [CrossRef]
- Ahmad, A.u.; Abbas, Q.; Ali, S.; Fakhar-e-alam, M.; Farooq, Z.; Farid, A.; Abbas, A.; Javid, M.; Muhammad Afzal, A.; Umair Arshad, H.M.; et al. Fluorinated hexagonal boron nitride as a spacer with silver nanorods for surface enhanced Raman spectroscopy analysis. Ceram. Int. 2021, 47, 6528–6534. [Google Scholar] [CrossRef]
- Jiang, J.; Zou, J.; Wee, A.T.S.; Zhang, W. Use of Single-Layer g-C3N4/Ag Hybrids for Surface-Enhanced Raman Scattering (SERS). Sci. Rep. 2016, 6, 34599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Sun, H.; Gu, C.; Zhang, Y.; Jiang, T. A hydrophilic–hydrophobic graphitic carbon nitride@silver hybrid substrate for recyclable surface-enhanced Raman scattering-based detection without the coffee-ring effect. Analyst 2021, 146, 5923–5933. [Google Scholar] [CrossRef]
- Tiwari, M.; Singh, A.; Thakur, D.; Pattanayek, S.K. Graphitic carbon nitride-based concoction for detection of melamine and R6G using surface-enhanced Raman scattering. Carbon 2022, 197, 311–323. [Google Scholar] [CrossRef]
- Rajput, P.; Devi, P. In-situ synthesized gold nanoparticles modified Mo2C MXene for surface enhanced Raman scattering. Graphene 2D Mater. 2022, 7, 107–117. [Google Scholar] [CrossRef]
- Soundiraraju, B.; George, B.K. Two-Dimensional Titanium Nitride (Ti2N) MXene: Synthesis, Characterization, and Potential Application as Surface-Enhanced Raman Scattering Substrate. ACS Nano 2017, 11, 8892–8900. [Google Scholar] [CrossRef]
- Satheeshkumar, E.; Makaryan, T.; Melikyan, A.; Minassian, H.; Gogotsi, Y.; Yoshimura, M. One-step Solution Processing of Ag, Au and Pd@MXene Hybrids for SERS. Sci. Rep. 2016, 6, 32049. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Cai, P.; Yang, L.; Liu, Y.; Zhu, L.; Zhang, Q.; Liu, J.; Huang, Z.; Yang, Y. Theoretical and Experimental Studies of Ti3C2 MXene for Surface-Enhanced Raman Spectroscopy-Based Sensing. ACS Omega 2020, 5, 26486–26496. [Google Scholar] [CrossRef]
- Lan, L.; Fan, X.; Yu, S.; Gao, J.; Zhao, C.; Hao, Q.; Qiu, T. Flexible Two-Dimensional Vanadium Carbide MXene-Based Membranes with Ultra-Rapid Molecular Enrichment for Surface-Enhanced Raman Scattering. ACS Appl. Mater. Interfaces 2022, 14, 40427–40436. [Google Scholar] [CrossRef]
- Barveen, N.R.; Wang, T.-J.; Chang, Y.H. A photochemical approach to anchor Au NPs on MXene as a prominent SERS substrate for ultrasensitive detection of chlorpromazine. Microchim. Acta 2021, 189, 16. [Google Scholar] [CrossRef]
- He, Z.; Rong, T.; Li, Y.; Ma, J.; Li, Q.; Wu, F.; Wang, Y.; Wang, F. Two-Dimensional TiVC Solid-Solution MXene as Surface-Enhanced Raman Scattering Substrate. ACS Nano 2022, 16, 4072–4083. [Google Scholar] [CrossRef]
- Shevchuk, K.; Sarycheva, A.; Gogotsi, Y. Evaluation of two-dimensional transition-metal carbides and carbonitrides (MXenes) for SERS substrates. MRS Bull. 2022, 47, 545–554. [Google Scholar] [CrossRef]
- Sun, H.; Gong, W.; Cong, S.; Liu, C.; Song, G.; Lu, W.; Zhao, Z. Ultrathin Two-Dimensional Metal–Organic Framework Nanosheets with Activated Ligand-Cluster Units for Enhanced SERS. ACS Appl. Mater. Interfaces 2022, 14, 2326–2334. [Google Scholar] [CrossRef]
- Zhang, G.-L.; Zhang, M.; Shi, Q.; Jiang, Z.; Tong, L.; Chen, Z.; Tang, B. In Situ Construction of COF-Based Paper Serving as a Plasmonic Substrate for Enhanced PSI-MS Detection of Polycyclic Aromatic Hydrocarbons. ACS Appl. Mater. Interfaces 2021, 13, 43438–43448. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, L.; Lee, W.B.; Ng, M.C. Structure of graphene and its disorders: A review. Sci. Technol. Adv. Mater. 2018, 19, 613–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Paidi, S.K.; Qin, Z.; Huang, Q.; Yu, C.-H.; Pagaduan, J.V.; Buehler, M.J.; Barman, I.; Gracias, D.H. Self-folding hybrid graphene skin for 3D biosensing. Nano Lett. 2018, 19, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-Y.; You, E.-M.; Tian, Z.Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef]
- Li, D.; Kaner, R.B. Graphene-based materials. Science 2008, 320, 1170–1171. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Q.; Shi, G. Graphene based new energy materials. Energy Environ. Sci. 2011, 4, 1113–1132. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lee, D.; Choi, J.-W. Bioelectronics. Live cell biosensing platforms using graphene-based hybrid nanomaterials. Biosens. Bioelectron. 2017, 94, 485–499. [Google Scholar] [CrossRef]
- Huang, S.; Pandey, R.; Barman, I.; Kong, J.; Dresselhaus, M. Raman enhancement of blood constituent proteins using graphene. ACS Photon. 2018, 5, 2978–2982. [Google Scholar] [CrossRef]
- Geng, Z.-Q.; Xu, D.; Song, Y.; Wang, W.-P.; Li, Y.-P.; Han, C.-Q.; Yang, G.-H.; Qu, L.-L.; Ajayan, P.M. Sensitive label-free detection of bilirubin in blood using boron nitride-modified nanorod arrays as SERS substrates. Sens. Actuators B Chem. 2021, 334, 129634. [Google Scholar] [CrossRef]
- Cai, Q.; Li, L.H.; Yu, Y.; Liu, Y.; Huang, S.; Chen, Y.; Watanabe, K.; Taniguchi, T. Boron nitride nanosheets as improved and reusable substrates for gold nanoparticles enabled surface enhanced Raman spectroscopy. Phys. Chem. Chem. Phys. 2015, 17, 7761–7766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.H.; Cervenka, J.; Watanabe, K.; Taniguchi, T.; Chen, Y. Strong oxidation resistance of atomically thin boron nitride nanosheets. ACS Nano 2014, 8, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Yan, X.; Yan, M.; Zeng, G.; Zhou, C.; Wan, J.; Cheng, M.; Xue, W. Graphitic carbon nitride-based heterojunction photoactive nanocomposites: Applications and mechanism insight. ACS Appl. Mater. Interfaces 2018, 10, 21035–21055. [Google Scholar] [CrossRef]
- Zhu, S.; Xiao, L.; Cortie, M.B. Surface enhanced Raman spectroscopy on metal nitride thin films. Vib. Spectrosc. 2016, 85, 146–148. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Z.; Wei, Y.; Wu, M.; Chen, Y.; Hu, S.; Pei, Y.; Cui, Y.; Lv, D.; Chen, Y.; et al. Synthesis and SERS activity of niobium nitride thin films via reduction nitridation of sol-gel derived films. Opt. Mater. 2022, 123, 111879. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Ye, G.J.; Ge, Q.; Ou, X.; Wu, H.; Feng, D.; Chen, X.H.; Zhang, Y.J. Black phosphorus field-effect transistors. Nat. Nanotechnol. 2014, 9, 372–377. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zheng, Y.; Rui, K.; Hng, H.H.; Hippalgaonkar, K.; Xu, J.; Sun, W.; Zhu, J.; Yan, Q.; Huang, W. 2D black phosphorus for energy storage and thermoelectric applications. Small 2017, 13, 1700661. [Google Scholar] [CrossRef]
- Sansone, G.; Maschio, L.; Usvyat, D.; Schütz, M.; Karttunen, A. Toward an Accurate Estimate of the Exfoliation Energy of Black Phosphorus: A Periodic Quantum Chemical Approach. J. Phys. Chem. Lett. 2016, 7, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.-C.; Liu, M.; Guo, Z.-N.; Jiang, X.-F.; Luo, A.-P.; Zhao, C.-J.; Yu, X.-F.; Xu, W.-C.; Zhang, H. Microfiber-based few-layer black phosphorus saturable absorber for ultra-fast fiber laser. Opt. Express 2015, 23, 20030–20039. [Google Scholar] [CrossRef]
- Lin, J.; Liang, L.; Ling, X.; Zhang, S.; Mao, N.; Zhang, N.; Sumpter, B.G.; Meunier, V.; Tong, L.; Zhang, J. Enhanced Raman Scattering on In-Plane Anisotropic Layered Materials. J. Am. Chem. Soc. 2015, 137, 15511–15517. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Nilghaz, A.; Lin, Y.; Xu, J.; Lu, X.J. Black phosphorus and its biomedical applications. Theranostics 2018, 8, 1005. [Google Scholar] [CrossRef] [PubMed]
- Gui, R.; Jin, H.; Wang, Z.; Li, J.J. Black phosphorus quantum dots: Synthesis, properties, functionalized modification and applications. Chem Soc Rev. 2018, 47, 6795–6823. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Qiao, H.; Huang, Z.; Ma, Q.; Liu, F.; Liao, G.; Luo, S.; Zhong, J.; Qi, X. Black Phosphorus Quantum Dots as Hole Capturers in Group-VA Monoelemental Heterostructures for the Application of High-Performance Flexible Photodetectors. ACS Sustain. Chem. Eng. 2021, 9, 14918–14926. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [Green Version]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Sarycheva, A.; Makaryan, T.; Maleski, K.; Satheeshkumar, E.; Melikyan, A.; Minassian, H.; Yoshimura, M.; Gogotsi, Y. Two-dimensional titanium carbide (MXene) as surface-enhanced Raman scattering substrate. J. Phys. Chem. C 2017, 121, 19983–19988. [Google Scholar] [CrossRef]
- Chhowalla, M.; Liu, Z.; Zhang, H. Two-dimensional transition metal dichalcogenide (TMD) nanosheets. Chem. Soc. Rev. 2015, 44, 2584–2586. [Google Scholar] [CrossRef]
- Ghopry, S.A.; Alamri, M.; Goul, R.; Cook, B.; Sadeghi, S.M.; Gutha, R.R.; Sakidja, R.; Wu, J.Z. Au Nanoparticle/WS2 Nanodome/Graphene van der Waals heterostructure substrates for surface-enhanced Raman spectroscopy. ACS Appl. Nano Mater. 2020, 3, 2354–2363. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Geim, A.K.; Grigorieva, I.V. Van der Waals heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, X.; Fang, W.; Lee, Y.-H.; Araujo, P.T.; Zhang, X.; Rodriguez-Nieva, J.F.; Lin, Y.; Zhang, J.; Kong, J.; Dresselhaus, M.S. Raman enhancement effect on two-dimensional layered materials: Graphene, h-BN and MoS2. Nano Lett. 2014, 14, 3033–3040. [Google Scholar] [CrossRef]
- Cong, S.; Yuan, Y.; Chen, Z.; Hou, J.; Yang, M.; Su, Y.; Zhang, Y.; Li, L.; Li, Q.; Geng, F.; et al. Noble metal-comparable SERS enhancement from semiconducting metal oxides by making oxygen vacancies. Nat. Commun. 2015, 6, 7800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Wang, H.; Li, G. Metal oxide semiconductor SERS-active substrates by defect engineering. Analyst 2017, 142, 326–335. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Lai, H.; Wang, S.; Chen, T.; Xie, F.; Chen, Q.; Liu, P.; Chen, J.; Xie, W. Few-layered vdW MoO3 for sensitive, uniform and stable SERS applications. Appl. Surf. Sci. 2020, 507, 145116. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, X.; Li, J.; Li, X.; Li, B.; Fei, W.; Zhou, J.; Yin, J.; Guo, W. Ultrathin Molybdenum Dioxide Nanosheets as Uniform and Reusable Surface-Enhanced Raman Spectroscopy Substrates with High Sensitivity. Small 2018, 14, 1802276. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, Y.; Peng, Y.; Huang, Z.; Ma, Q.; Zhang, H. Two-dimensional metal–organic framework nanosheets: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 6267–6295. [Google Scholar] [CrossRef]
- Carrasco, S. Metal-Organic Frameworks for the Development of Biosensors: A Current Overview. Biosensors 2018, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Yu, B.; Pan, X.; Zhu, X.; Liu, Z. Recent progress in metal–organic frameworks-based materials toward surface-enhanced Raman spectroscopy. Appl. Spectrosc. Rev. 2022, 57, 513–528. [Google Scholar] [CrossRef]
- Yu, T.-H.; Ho, C.-H.; Wu, C.-Y.; Chien, C.-H.; Lin, C.-H.; Lee, S. Metal–organic frameworks: A novel SERS substrate. J. Raman Spectrosc. 2013, 44, 1506–1511. [Google Scholar] [CrossRef]
- Sun, H.; Cong, S.; Zheng, Z.; Wang, Z.; Chen, Z.; Zhao, Z. Metal–Organic Frameworks as Surface Enhanced Raman Scattering Substrates with High Tailorability. J. Am. Chem. Soc. 2019, 141, 870–878. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Singh, E.; Singh, P.; Meyyappan, M.; Nalwa, H.S. A review on graphene-based nanocomposites for electrochemical and fluorescent biosensors. RSC Adv. 2019, 9, 8778–8881. [Google Scholar] [PubMed]
- Ganesh, B.M.; Isloor, A.M.; Ismail, A.F. Enhanced hydrophilicity and salt rejection study of graphene oxide-polysulfone mixed matrix membrane. Desalination 2013, 313, 199–207. [Google Scholar] [CrossRef]
- Lee, J.; Chae, H.R.; Won, Y.J.; Lee, K.; Lee, C.H.; Lee, H.H.; Kim, I.-C.; Lee, J.M. Graphene oxide nanoplatelets composite membrane with hydrophilic and antifouling properties for wastewater treatment. J. Membr. Sci. 2013, 448, 223–230. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Kim, S.; Min, D.H. Biosensors based on graphene oxide and its biomedical application. Adv. Drug Deliv. Rev. 2016, 105, 275–287. [Google Scholar] [CrossRef]
- Lan, L.; Yao, H.; Li, G.; Fan, X.; Li, M.; Qiu, T. Structural engineering of transition-metal nitrides for surface-enhanced Raman scattering chips. Nano Res. 2022, 15, 3794–3803. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, H.; Li, X.; Chen, Y.; Gu, C.; Wei, G.; Zhou, J.; Jiang, T. Nonmetallic SERS-based immunosensor byintegrating MoS2 nanoflower and nanosheet towards the direct serum detection of carbohydrate antigen 19-9. Biosens. Bioelectron. 2021, 193, 113481. [Google Scholar] [CrossRef]
- Peng, Y.; Lin, C.; Long, L.; Masaki, T.; Tang, M.; Yang, L.; Liu, J.; Huang, Z.; Li, Z.; Luo, X.; et al. Charge-Transfer Resonance and Electromagnetic Enhancement Synergistically Enabling MXenes with Excellent SERS Sensitivity for SARS-CoV-2 S Protein Detection. Nano-Micro Lett. 2021, 13, 52. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Wei, B. Hybrid nanostructures of metal/two-dimensional nanomaterials for plasmon-enhanced applications. Chem. Soc. Rev. 2016, 45, 3145–3187. [Google Scholar] [CrossRef]
- Kamat, P.V. Graphene-Based Nanoarchitectures. Anchoring Semiconductor and Metal Nanoparticles on a Two-Dimensional Carbon Support. J. Phys. Chem. Lett. 2010, 1, 520–527. [Google Scholar] [CrossRef]
- He, S.; Liu, K.-K.; Su, S.; Yan, J.; Mao, X.; Wang, D.; He, Y.; Li, L.-J.; Song, S.; Fan, C. Graphene-Based High-Efficiency Surface-Enhanced Raman Scattering-Active Platform for Sensitive and Multiplex DNA Detection. Anal. Chem. 2012, 84, 4622–4627. [Google Scholar] [CrossRef]
- Fan, Z.; Kanchanapally, R.; Ray, P.C. Hybrid Graphene Oxide Based Ultrasensitive SERS Probe for Label-Free Biosensing. J. Phys. Chem. Lett. 2013, 4, 3813–3818. [Google Scholar] [CrossRef]
- Khalil, I.; Yehye, W.A.; Julkapli, N.M.; Rahmati, S.; Sina, A.A.; Basirun, W.J.; Johan, M.R. Graphene oxide and gold nanoparticle based dual platform with short DNA probe for the PCR free DNA biosensing using surface-enhanced Raman scattering. Biosens. Bioelectron. 2019, 131, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Khalil, I.; Yehye, W.A.; Muhd Julkapli, N.; Ibn Sina, A.A.; Islam Chowdhury, F.; Khandaker, M.U.; Hsiao, V.K.S.; Basirun, W.J. Simultaneous detection of dual food adulterants using graphene oxide and gold nanoparticle based surface enhanced Raman scattering duplex DNA biosensor. Vib. Spectrosc. 2021, 116, 103293. [Google Scholar] [CrossRef]
- Kim, G.; Kim, J.; Kim, S.M.; Kato, T.; Yoon, J.; Noh, S.; Park, E.Y.; Park, C.; Lee, T.; Choi, J.W. Fabrication of MERS-nanovesicle biosensor composed of multi-functional DNA aptamer/graphene-MoS2 nanocomposite based on electrochemical and surface-enhanced Raman spectroscopy. Sens. Actuators B Chem. 2022, 352, 131060. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, C.; Cui, M.; Zhang, X.; Yang, D.-P.; Wang, X.; Cui, D. Graphene oxide wrapped with gold nanorods as a tag in a SERS based immunoassay for the hepatitis B surface antigen. Mikrochim. Acta 2018, 185, 1–8. [Google Scholar] [CrossRef]
- Yang, L.; Zhen, S.J.; Li, Y.F.; Huang, C.Z. Silver nanoparticles deposited on graphene oxide for ultrasensitive surface-enhanced Raman scattering immunoassay of cancer biomarker. Nanoscale 2018, 10, 11942–11947. [Google Scholar] [CrossRef]
- Zeng, F.; Xu, D.; Zhan, C.; Liang, C.; Zhao, W.; Zhang, J.; Feng, H.; Ma, X. Surfactant-Free Synthesis of Graphene Oxide Coated Silver Nanoparticles for SERS Biosensing and Intracellular Drug Delivery. ACS Appl. Nano Mater. 2018, 1, 2748–2753. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Z.; Zhao, J.; Yu, Z.; Jiang, H. Raman spectroscopy study of the effect of urea, uric acid and creatinine on steric configuration of bovine hemoglobin using SERS-active BN nanosheets/Ag nanoparticles hybrids. Mater. Lett. 2014, 131, 78–81. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, H.; Jia, Y.; Zhang, W.; Zhao, H.; Fan, W.; Zhang, W.; Zhong, H.; Ni, Y.; Guo, Z. A two-dimensional fingerprint nanoprobe based on black phosphorus for bio-SERS analysis and chemo-photothermal therapy. Nanoscale 2018, 10, 18795–18804. [Google Scholar] [CrossRef]
- Fang, X.; Song, Y.; Huang, Y.; Yang, G.; Han, C.; Li, H.; Qu, L. Two-dimensional MXene modified AgNRs as a surface-enhanced Raman scattering substrate for sensitive determination of polychlorinated biphenyls. Analyst 2020, 145, 7421–7428. [Google Scholar] [CrossRef]
- Liu, L.; Shangguan, C.; Guo, J.; Ma, K.; Jiao, S.; Yao, Y.; Wang, J. Ultrasensitive SERS Detection of Cancer-Related miRNA-182 by MXene/MoS2@AuNPs with Controllable Morphology and Optimized Self-Internal Standards. Adv. Opt. Mater. 2020, 8, 2001214. [Google Scholar] [CrossRef]
- Medetalibeyoglu, H.; Kotan, G.; Atar, N.; Yola, M.L. A novel sandwich-type SERS immunosensor for selective and sensitive carcinoembryonic antigen (CEA) detection. Anal. Chim. Acta 2020, 1139, 100–110. [Google Scholar] [CrossRef]
- Zheng, F.; Ke, W.; Shi, L.; Liu, H.; Zhao, Y. Plasmonic Au–Ag Janus Nanoparticle Engineered Ratiometric Surface-Enhanced Raman Scattering Aptasensor for Ochratoxin A Detection. Anal. Chem. 2019, 91, 11812–11820. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, P.; Xue, T.; Ge, Y.; Ai, S.; Sheng, Y.; Wu, R.; Xu, L.; Tang, K.; Wen, Y. A novel graphene-like titanium carbide MXene/Au–Ag nanoshuttles bifunctional nanosensor for electrochemical and SERS intelligent analysis of ultra-trace carbendazim coupled with machine learning. Ceram. Int. 2021, 47, 173–184. [Google Scholar] [CrossRef]
- Singha, S.S.; Mondal, S.; Bhattacharya, T.S.; Das, L.; Sen, K.; Satpati, B.; Das, K.; Singha, A. Au nanoparticles functionalized 3D-MoS2 nanoflower: An efficient SERS matrix for biomolecule sensing. Biosens. Bioelectron. 2018, 119, 10–17. [Google Scholar] [CrossRef]
- Shorie, M.; Kumar, V.; Kaur, H.; Singh, K.; Tomer, V.K.; Sabherwal, P. Plasmonic DNA hotspots made from tungsten disulfide nanosheets and gold nanoparticles for ultrasensitive aptamer-based SERS detection of myoglobin. Microchim. Acta 2018, 185, 158. [Google Scholar] [CrossRef]
- Hu, S.; Jiang, Y.; Wu, Y.; Guo, X.; Ying, Y.; Wen, Y.; Yang, H. Enzyme-Free Tandem Reaction Strategy for Surface-Enhanced Raman Scattering Detection of Glucose by Using the Composite of Au Nanoparticles and Porphyrin-Based Metal–Organic Framework. ACS Appl. Mater. Interfaces 2020, 12, 55324–55330. [Google Scholar] [CrossRef]
- Lai, H.; Dai, H.; Li, G.; Zhang, Z. Rapid determination of pesticide residues in fruit and vegetable using Au@AgNPs decorated 2D Ni-MOF nanosheets as efficient surface-enhanced Raman scattering substrate. Sens. Actuators B Chem. 2022, 369, 132360. [Google Scholar] [CrossRef]
- He, J.; Xu, F.; Chen, Z.; Hou, X.; Liu, Q.; Long, Z. AuNPs/COFs as a new type of SERS substrate for sensitive recognition of polyaromatic hydrocarbons. Chem. Commun. 2017, 53, 11044–11047. [Google Scholar] [CrossRef] [PubMed]
- Silver, A.; Kitadai, H.; Liu, H.; Granzier-Nakajima, T.; Terrones, M.; Ling, X.; Huang, S. Chemical and Bio Sensing Using Graphene-Enhanced Raman Spectroscopy. Nanomaterials 2019, 9, 516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Jin, M.; Wu, W.; Fu, L.; Yin, C.; Karimi-Maleh, H. Graphene-Based Surface-Enhanced Raman Scattering (SERS) Sensing: Bibliometrics Based Analysis and Review. Chemosensors 2022, 10, 317. [Google Scholar] [CrossRef]
- Xu, W.; Ling, X.; Xiao, J.; Dresselhaus, M.S.; Kong, J.; Xu, H.; Liu, Z.; Zhang, J. Surface enhanced Raman spectroscopy on a flat graphene surface. Proc. Natl. Acad. Sci. USA 2012, 109, 9281–9286. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.M.; Ling, X.; Fang, Y.; Zhang, J.; Liu, Z.F. Graphene as a Substrate To Suppress Fluorescence in Resonance Raman Spectroscopy. J. Am. Chem. Soc. 2009, 131, 9890–9891. [Google Scholar] [CrossRef] [PubMed]
- Liang, O.; Wang, P.; Xia, M.; Augello, C.; Yang, F.; Niu, G.; Liu, H.; Xie, Y.-H. Label-free distinction between p53+/+ and p53-/-colon cancer cells using a graphene based SERS platform. Biosens. Bioelectron. 2018, 118, 108–114. [Google Scholar] [CrossRef]
- Demeritte, T.; Viraka Nellore, B.P.; Kanchanapally, R.; Sinha, S.S.; Pramanik, A.; Chavva, S.R.; Ray, P.C. Hybrid Graphene Oxide Based Plasmonic-Magnetic Multifunctional Nanoplatform for Selective Separation and Label-Free Identification of Alzheimer’s Disease Biomarkers. ACS Appl. Mater. Interfaces 2015, 7, 13693–13700. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Zhao, D.; Ding, M.; Zhao, H.; Zhang, Z.; Li, B.; Shen, D. A white-emitting ZnO–Au nanocomposite and its SERS applications. Appl. Surf. Sci. 2012, 258, 7813–7819. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Xie, J. Graphene in Photocatalysis: A Review. Small 2016, 12, 6640–6696. [Google Scholar] [CrossRef]
- Yang, L.; Lee, J.-H.; Rathnam, C.; Hou, Y.; Choi, J.-W.; Lee, K.-B. Dual-Enhanced Raman Scattering-Based Characterization of Stem Cell Differentiation Using Graphene-Plasmonic Hybrid Nanoarray. Nano Lett. 2019, 19, 8138–8148. [Google Scholar] [CrossRef]
- Choi, J.-H.; Kim, T.-H.; El-said, W.A.; Lee, J.-H.; Yang, L.; Conley, B.; Choi, J.-W.; Lee, K.-B. In Situ Detection of Neurotransmitters from Stem Cell-Derived Neural Interface at the Single-Cell Level via Graphene-Hybrid SERS Nanobiosensing. Nano Lett. 2020, 20, 7670–7679. [Google Scholar] [CrossRef]
- Cui, X.; Lee, G.-H.; Kim, Y.D.; Arefe, G.; Huang, P.Y.; Lee, C.-H.; Chenet, D.A.; Zhang, X.; Wang, L.; Ye, F. Multi-terminal transport measurements of MoS2 using a van der Waals heterostructure device platform. Nat. Nanotechnol. 2015, 10, 534–540. [Google Scholar] [CrossRef]
- Wang, J.I.-J.; Yang, Y.; Chen, Y.-A.; Watanabe, K.; Taniguchi, T.; Churchill, H.O.; Jarillo-Herrero, P. Electronic transport of encapsulated graphene and WSe2 devices fabricated by pick-up of prepatterned hBN. Nano Lett. 2015, 15, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Britnell, L.; Gorbachev, R.; Jalil, R.; Belle, B.; Schedin, F.; Mishchenko, A.; Georgiou, T.; Katsnelson, M.; Eaves, L.; Morozov, S. Field-effect tunneling transistor based on vertical graphene heterostructures. Science 2012, 335, 947–950. [Google Scholar] [CrossRef] [Green Version]
- Dai, P.; Xue, Y.; Wang, X.; Weng, Q.; Zhang, C.; Jiang, X.; Tang, D.; Wang, X.; Kawamoto, N.; Ide, Y. Pollutant capturing SERS substrate: Porous boron nitride microfibers with uniform silver nanoparticle decoration. Nanoscale 2015, 7, 18992–18997. [Google Scholar] [CrossRef]
- Cai, Q.; Mateti, S.; Yang, W.; Jones, R.; Watanabe, K.; Taniguchi, T.; Huang, S.; Chen, Y.; Li, L.H. Boron Nitride Nanosheets Improve Sensitivity and Reusability of Surface-Enhanced Raman Spectroscopy. Angew. Chem. Int. Ed. 2016, 55, 8405–8409. [Google Scholar] [CrossRef] [Green Version]
- Cai, Q.; Mateti, S.; Watanabe, K.; Taniguchi, T.; Huang, S.; Chen, Y.; Li, L.H. Boron Nitride Nanosheet-Veiled Gold Nanoparticles for Surface-Enhanced Raman Scattering. ACS Appl. Mater. Interfaces 2016, 8, 15630–15636. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.; Kim, M.; Hyun, C.; Hong, S.; Ma, K.Y.; Shin, H.S.; Lim, H. Hexagonal Boron Nitride/Au Substrate for Manipulating Surface Plasmon and Enhancing Capability of Surface-Enhanced Raman Spectroscopy. ACS Nano 2016, 10, 11156–11162. [Google Scholar] [CrossRef]
- Pakdel, A.; Bando, Y.; Golberg, D. Nano boron nitride flatland. Chem. Soc. Rev. 2014, 43, 934–959. [Google Scholar] [CrossRef]
- Majdoub, M.; Anfar, Z.; Amedlous, A. Emerging Chemical Functionalization of g-C3N4: Covalent/Noncovalent Modifications and Applications. ACS Nano 2020, 14, 12390–12469. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-N.; Zhang, Y.; Zhang, W.-S.; Xu, Z.-R. A SERS substrate of mesoporous g-C3N4 embedded with in situ grown gold nanoparticles for sensitive detection of 6-thioguanine. Sens. Actuators B Chem. 2018, 260, 400–407. [Google Scholar] [CrossRef]
- Wang, J.; Liu, R.; Zhang, C.; Han, G.; Zhao, J.; Liu, B.; Jiang, C.; Zhang, Z. Synthesis of g-C3N4 nanosheet/Au@Ag nanoparticle hybrids as SERS probes for cancer cell diagnostics. RSC Adv. 2015, 5, 86803–86810. [Google Scholar] [CrossRef]
- Wang, C.; Cui, Q.; Yue, T.; Jiao, A.; Ma, H.; Zhang, M.; Zheng, L.; Li, S.; Li, G.; Chen, M. Thermal annealing-boosted photoinduced electron transfer efficiency of g-C3N4/Au NPs hybrids for promoting SERS detection of uric acids. Vib. Spectrosc. 2022, 122, 103424. [Google Scholar] [CrossRef]
- Yang, G.; Liu, Z.; Li, Y.; Hou, Y.; Fei, X.; Su, C.; Wang, S.; Zhuang, Z.; Guo, Z. Facile synthesis of black phosphorus–Au nanocomposites for enhanced photothermal cancer therapy and surface-enhanced Raman scattering analysis. Biomater. Sci. 2017, 5, 2048–2055. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Neal, A.T.; Zhu, Z.; Luo, Z.; Xu, X.; Tománek, D.; Ye, P.D. Phosphorene: An Unexplored 2D Semiconductor with a High Hole Mobility. ACS Nano 2014, 8, 4033–4041. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Jiang, L.; Yu, Z.; Jing, X.; Liang, X.; Wang, D.; Yang, B.; Lu, C.; Zhou, W.; Jin, S. MXene (Ti3C2Tx)-Ag nanocomplex as efficient and quantitative SERS biosensor platform by in-situ PDDA electrostatic self-assembly synthesis strategy. Sens. Actuators B Chem. 2021, 333, 129581. [Google Scholar] [CrossRef]
- Wei, W.; Lin, H.; Hao, T.; Su, X.; Jiang, X.; Wang, S.; Hu, Y.; Guo, Z. Dual-mode ECL/SERS immunoassay for ultrasensitive determination of Vibrio vulnificus based on multifunctional MXene. Sens. Actuators B Chem. 2021, 332, 129525. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Zeng, H.; Cui, X. An optical spectroscopic study on two-dimensional group-VI transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2629–2642. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, J.; Wang, F.; Xiong, Y.; Wu, Y.; Wang, Q.; Weng, J.; Zhang, Z.; Chen, W.; Liu, S. Improved In Vitro and In Vivo Biocompatibility of Graphene Oxide through Surface Modification: Poly(Acrylic Acid)-Functionalization is Superior to PEGylation. ACS Nano 2016, 10, 3267–3281. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Hong, H.; Cai, W.; Liu, Z. Preparation and functionalization of graphene nanocomposites for biomedical applications. Nat. Protoc. 2013, 8, 2392–2403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bullock, C.J.; Bussy, C. Biocompatibility Considerations in the Design of Graphene Biomedical Materials. Adv. Mater. Interfaces 2019, 6, 1900229. [Google Scholar] [CrossRef]
- Agarwal, V.; Chatterjee, K. Recent advances in the field of transition metal dichalcogenides for biomedical applications. Nanoscale 2018, 10, 16365–16397. [Google Scholar] [CrossRef]
- Derakhshi, M.; Daemi, S.; Shahini, P.; Habibzadeh, A.; Mostafavi, E.; Ashkarran, A.A. Two-Dimensional Nanomaterials beyond Graphene for Biomedical Applications. J. Funct. Biomater. 2022, 13, 27. [Google Scholar] [CrossRef] [PubMed]







| 2DNS-SERS Substrate | Probe Molecules | Mechanism | EF | Ref. |
|---|---|---|---|---|
| Graphene | ||||
| Graphene | Rh6G | CM | 1.7 to 5.6 | [32] |
| UV/Ozone-GO | RhB, Rh6G, and CV | CM | ∼104 | [33] |
| rGO | Rh6G | CM | ∼103 | [34] |
| AgNPs/rGO | Rh6G | CM + EM | 2.3 × 108 | [35] |
| AuNPs/GO/CW | Rh6G | CM + EM | 1.0 × 106 | [36] |
| AgNPs/rGO | RhB | CM + EM | 2.0 × 107 | [37] |
| AuNPs/rGO/ | MG | CM + EM | 3.8 × 103 | [38] |
| AgNPs/CVD Graphene | Rh6G | CM + EM | ∼109 | [39] |
| TMD | ||||
| TiS2 | Rh6G | CM | 3.2 × 105 | [40] |
| 1T-W(MoTe2) | Rh6G | CM | 1.8 × 109 | [41] |
| 2H-TaS2 | Rh6G | CM | 1.3 × 1014 | [42] |
| Oxygen incorporated MoS2 | Rh6G | CM | 1.4 × 105 | [43] |
| MoTe2 | β-sitosterol | CM | 1.3 × 104 | [44] |
| HfTe2 | Rh6G, CV, MB, and MG | CM | ∼106 | [45] |
| AuNPs/HfTe2 | MB | CM + EM | 1.7 × 108 | [46] |
| AuNWs/MoS2 | Rh6G and MB | CM + EM | ∼107 | [47] |
| Black phosphorous (BP) | ||||
| BPQDs/AgNPs/TiO2 | 4-MBA | CM + EM | 2.5 × 105 | [48] |
| BP flakes | RhB | CM | ∼106 | [49] |
| BP Nanosheets | Rh6G | CM | 6.7 × 107 | [50] |
| AgNPs/BP | Interleukin-3 (IL-3) and procalcitonin (PCT) | CM + EM | ∼1014 | [51] |
| Nitride | ||||
| Hexagonal Boron Nitride (h-BN) | MG, MB and Rh6G | CM | ∼104 | [52] |
| Fluorinated h-BN | Rh6G and CV | CM | ∼108 | [53] |
| AgNPs/g-C3N4 | CV | CM + EM | 2.1 × 109 | [54] |
| Hydrophilic hydrophobic g-C3N4@Ag | MG | CM + EM | 3.2 × 106 | [55] |
| AuNPs/g-C3N4 | Rh6G and Melamine | CM + EM | ∼108 | [56] |
| MXenes | ||||
| AuNPs/Mo2C MXene | MB | CM + EM | 2.2 × 104 | [57] |
| Ti2N MXene | Rh6G | CM | ∼1012 | [58] |
| Ti3C2 | MB | CM | ∼105 | [59] |
| Ti3C2 MXene | MB | CM | 2.9 × 106 | [60] |
| V4C3 and V2C | Rh6G | CM | ∼105 | [61] |
| AuNPs/TiC | Chlorpromazine | CM + EM | ∼109 | [62] |
| TiVC | Rh6G | CM | 3.3 × 1012 | [63] |
| Nb2C, Mo2C, Ti2C, V2C, Ti3C2, Mo2TiC2, and Ti3CN | Rh6G | CM | - | [64] |
| 2D MOFs/COFs | ||||
| Co-MOFs | Rh6G | CM | - | [65] |
| AuNPs/COF-paper | PAHs | CM + EM | 12 to 194 | [66] |
| 2DNS Support | Nanoparticle | Sample | Target | LOD | Ref. |
|---|---|---|---|---|---|
| Graphene-supported SERS substrates | |||||
| Graphene | AuNPs | DNA oligonucleotides | DNA | 10 × 10−12 M | [115] |
| GO | popcorn-shaped AuNPs | Culture Collection | HIV DNA and bacteria | 10 CFU/mL | [116] |
| GO | AuNPs | DNA sequence | DNA | 10 × 10−15 M | [117] |
| GO | AuNPs | muscle tissue of the MBT and pork samples | DNA | 10−14 M | [118] |
| GO (encapsulated) | AuNPs | saliva | MERS-CoV | 0.525 pg/mL | [119] |
| GO | Au nanorods | serum | hepatitis B surface antigen | 0.05 pg/mL | [120] |
| GO | AuNPs | serum | prostate-specific antigen | 0.23 pg/mL | [121] |
| GO | AuNPs | Hep-G2 liver cancer cells | doxorubicin | - | [122] |
| Nitride-supported SERS substrates | |||||
| BN | Ag nanoarrays | blood | bilirubin | 2.5 × 10−8 M | [74] |
| BN | AgNPs | bovine haemoglobin | urea, uric acid and creatinine | - | [123] |
| Black Phosphorous-supported SERS substrates | |||||
| multi-layer BP | AgNPs | Human lung carcinoma | Exosome | - | [50] |
| BP | AuNPs | Hep-G2 live cell | Hep-G2 cells | - | [124] |
| BP | AgNPs | Serum | LPS, IL-3, and PCT | 10−9 M, 10−12 M and 10−13 M | [51] |
| MXenes-supported SERS substrates | |||||
| MXene | Ag nanorods | soil | PCB-77 and PCB-3 | 2 × 10−10 M and 2 × 10−9 M | [125] |
| MXene/MoS2 | AuNPs | human serum | miRNA-182 | 6.6 × 10−10 M | [126] |
| Ti3C2Tx MXene | AuNPs | serum | adenine | 10−8 M | [87] |
| MXene/MoS2 | AuNPs | Bovine serum albumin | carcinoembryonic antigen | 0.033 pg/mL | [127] |
| Nb2C and Ta2C MXenes | SARS-CoV-2 | 5 × 10−9 M, | [112] | ||
| MXenes | Au−Ag NPs | bovine serum albumin | Ochratoxin A | 1.3 × 10−12 M | [128] |
| Ti2C MXene | Au–Ag NPs | food | carbendazim | 0.01 × 10−6 M | [129] |
| TMD-supported SERS substrates | |||||
| MoS2 | - | serum | CA19-9 | 3.4 × 10−4 IU/mL | [111] |
| MoS2 | AuNPs | serum | bilirubin | 10−12 M | [130] |
| MoTe2 | Ag nanorods | Phosphate buffered saline | β-sitosterol | 10−9 M | [44] |
| HfTe2 | - | - | uric acid | 0.1 × 10−6 M | [45] |
| HfTe2 | AuNPs | - | foodborne pathogenic bacteria | 10 CFU/mL | [46] |
| WS2 | AuNPs | serum | cardiac marker myoglobin | 0.5 × 10−18 M | [131] |
| 2D MOF/COF-supported SERS substrates | |||||
| Cu-TCPP(Fe) | AuNPs | Saliva | Glucose | 3.9 × 10−6 M | [132] |
| Ni-MOF | Au@AgNPs | - | thiram, diquat, and paraquat | 87.1, 188.8, and 8.9 μg/L | [133] |
| COFs | AuNPs | - | PAHs | - | [134] |
| 2DNS | Common Preparation Method | Enhancement Mechanism | Typical EF | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Graphene, GO | Exfoliation, CVD | CM | ≤103 | Easy preparation, lower cost, and biocompatibility. | Low EF |
| Nitride (h-BN, g-C3N4) | Exfoliation, CVD | CM | ≤104 | Thermal conductivity, mechanical, chemical and thermal stability | Low EF |
| BP | Exfoliation | CM | ≤106 | Higher surface-to-volume ratio, anisotropy, low toxicity | Tendency of oxidation |
| MXenes | Chemical etching delamination | CM + EM | ≥106 | Highest enhancement for a 2DNS, low toxicity | Tendency of oxidation, harsh preparation conditions |
| TMD | Exfoliation, CVD | CM | ≤106 | Tunable bandgap, layer-dependent behaviour, high stability | Phase transition decreasing EF |
| 2D MOF/COF | Chemical synthesis | CM | ≤106 | large specific surface area, easy customization, biocompatibility | Poor stability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jebakumari, K.A.E.; Murugasenapathi, N.K.; Palanisamy, T. Engineered Two-Dimensional Nanostructures as SERS Substrates for Biomolecule Sensing: A Review. Biosensors 2023, 13, 102. https://doi.org/10.3390/bios13010102
Jebakumari KAE, Murugasenapathi NK, Palanisamy T. Engineered Two-Dimensional Nanostructures as SERS Substrates for Biomolecule Sensing: A Review. Biosensors. 2023; 13(1):102. https://doi.org/10.3390/bios13010102
Chicago/Turabian StyleJebakumari, K. A. Esther, N. K. Murugasenapathi, and Tamilarasan Palanisamy. 2023. "Engineered Two-Dimensional Nanostructures as SERS Substrates for Biomolecule Sensing: A Review" Biosensors 13, no. 1: 102. https://doi.org/10.3390/bios13010102
APA StyleJebakumari, K. A. E., Murugasenapathi, N. K., & Palanisamy, T. (2023). Engineered Two-Dimensional Nanostructures as SERS Substrates for Biomolecule Sensing: A Review. Biosensors, 13(1), 102. https://doi.org/10.3390/bios13010102