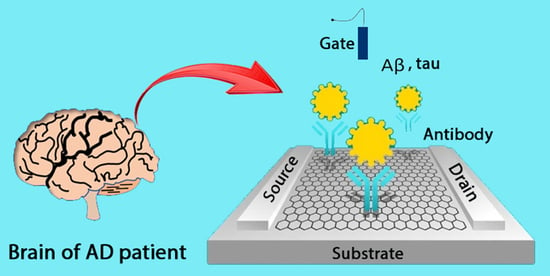
Alzheimer’s Disease Biomarker Detection Using Field Effect Transistor-Based Biosensor
Abstract
:1. Introduction
2. Overview of the FET Biosensor
2.1. The FET Biosensor Architecture
2.2. Nanomaterial Preparation for FET Sensor Fabrication
2.3. Overview of Sensing Mechanism of FET Biosensor
2.4. Responsive Signal of FET Biosensor
3. Application of FET Biosensors in AD Biomarker Detection
3.1. AD Biomarker Detection Methodology
3.2. Recent Research Progress in AD Biomarker Detection
3.2.1. Architecture of a Fabricated FET Biosensor through Recent Representative Research
3.2.2. AD Biomarker Detection via Representative Research
3.2.3. Signal Response of FET Biosensor via Representative Research
4. Conclusions and Future Vision
Author Contributions
Funding
Conflicts of Interest
References
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Le, P.G.; Le, H.T.N.; Kim, H.-E.; Cho, S. SAM-Support-Based Electrochemical Sensor for Aβ Biomarker Detection of Alzheimer’s Disease. Biosensors 2023, 13, 809. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-F.; Xu, T.-H.; Yan, Y.; Zhou, Y.-R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Mirzaie, A.; Nasrollahpour, H.; Khalilzadeh, B.; Jamali, A.A.; Spiteri, R.J.; Yousefi, H.; Isildak, I.; Rahbarghazi, R. Cerebrospinal fluid: A specific biofluid for the biosensing of Alzheimer’s diseases biomarkers. TrAC Trends Anal. Chem. 2023, 166, 117174. [Google Scholar] [CrossRef]
- Ding, M.; Ding, S.; Du, D.; Wang, X.; Hu, X.; Guan, P.; Lyu, Z.; Lin, Y. Recent advances in electrochemical biosensors for the detection of Aβ42, a biomarker for Alzheimer disease diagnosis. TrAC Trends Anal. Chem. 2023, 164, 117087. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; van der Kant, R.; Hansson, O. Tau biomarkers in Alzheimer’s disease: Towards implementation in clinical practice and trials. Lancet Neurol. 2022, 21, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Soylemez, S.; Dolgun, V.; Özçubukçu, S. Fullerene-based mimics of enhanced acetylcholine detection for the diagnosis of Alzheimer’s disease. Microchem. J. 2023, 193, 109099. [Google Scholar] [CrossRef]
- Chae, M.-S.; Yoo, Y.K.; Kim, J.; Kim, T.G.; Hwang, K.S. Graphene-based enzyme-modified field-effect transistor biosensor for monitoring drug effects in Alzheimer’s disease treatment. Sens. Actuators B Chem. 2018, 272, 448–458. [Google Scholar] [CrossRef]
- Ricci, S.; Casalini, S.; Parkula, V.; Selvaraj, M.; Saygin, G.D.; Greco, P.; Biscarini, F.; Mas-Torrent, M. Label-free immunodetection of α-synuclein by using a microfluidics coplanar electrolyte-gated organic field-effect transistor. Biosens. Bioelectron. 2020, 167, 112433. [Google Scholar] [CrossRef]
- Blennow, K.; Dubois, B.; Fagan, A.M.; Lewczuk, P.; de Leon, M.J.; Hampel, H. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 58–69. [Google Scholar] [CrossRef]
- Benzinger, T.L.S.; Blazey, T.; Jack, C.R.; Koeppe, R.A.; Su, Y.; Xiong, C.; Raichle, M.E.; Snyder, A.Z.; Ances, B.M.; Bateman, R.J.; et al. Regional variability of imaging biomarkers in autosomal dominant Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2013, 110, E4502–E4509. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Kovac, A.; Majerova, P.; Bullock, K.M.; Shi, M.; Zhang, J. Tau Proteins Cross the Blood-Brain Barrier. J. Alzheimer’s Dis. 2017, 55, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Kutovyi, Y.; Hlukhova, H.; Boichuk, N.; Menger, M.; Offenhäusser, A.; Vitusevich, S. Amyloid-beta peptide detection via aptamer-functionalized nanowire sensors exploiting single-trap phenomena. Biosens. Bioelectron. 2020, 154, 112053. [Google Scholar] [CrossRef] [PubMed]
- Vergallo, A.; Bun, R.-S.; Toschi, N.; Baldacci, F.; Zetterberg, H.; Blennow, K.; Cavedo, E.; Lamari, F.; Habert, M.-O.; Dubois, B.; et al. Association of cerebrospinal fluid α-synuclein with total and phospho-tau181 protein concentrations and brain amyloid load in cognitively normal subjective memory complainers stratified by Alzheimer’s disease biomarkers. Alzheimer’s Dement. 2018, 14, 1623–1631. [Google Scholar] [CrossRef]
- Twohig, D.; Rodriguez-Vieitez, E.; Sando, S.B.; Berge, G.; Lauridsen, C.; Møller, I.; Grøntvedt, G.R.; Bråthen, G.; Patra, K.; Bu, G.; et al. The relevance of cerebrospinal fluid α-synuclein levels to sporadic and familial Alzheimer’s disease. Acta Neuropathol. Commun. 2018, 6, 130. [Google Scholar] [CrossRef]
- Bullich, S.; Seibyl, J.; Catafau, A.M.; Jovalekic, A.; Koglin, N.; Barthel, H.; Sabri, O.; De Santi, S. Optimized classification of 18F-Florbetaben PET scans as positive and negative using an SUVR quantitative approach and comparison to visual assessment. NeuroImage Clin. 2017, 15, 325–332. [Google Scholar] [CrossRef]
- Nikiforova, A.; Sedov, I. Molecular Probes for Magnetic Resonance Imaging of Amyloid β Peptides. Int. J. Mol. Sci. 2023, 24, 11152. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Kang, J.; Ye, T.; Yang, Z.; Liu, Z.; Liu, Q.; Zhao, Y.; Liu, G.; Pan, J. Application of a novel coumarin-derivative near-infrared fluorescence probe to amyloid-β imaging and inhibition in Alzheimer’s disease. J. Lumin. 2023, 256, 119661. [Google Scholar] [CrossRef]
- Song, Y.; Xu, T.; Zhu, Q.; Zhang, X. Integrated individually electrochemical array for simultaneously detecting multiple Alzheimer’s biomarkers. Biosens. Bioelectron. 2020, 162, 112253. [Google Scholar] [CrossRef]
- Liao, X.; Ge, K.; Cai, Z.; Qiu, S.; Wu, S.; Li, Q.; Liu, Z.; Gao, F.; Tang, Q. Hybridization chain reaction triggered poly adenine to absorb silver nanoparticles for label-free electrochemical detection of Alzheimer’s disease biomarkers amyloid β-peptide oligomers. Anal. Chim. Acta 2022, 1192, 339391. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, Y.; Xu, T.; Cheng, G.; Cai, H.; Zhang, X. Acoustic aggregation-induced separation for enhanced fluorescence detection of Alzheimer’s biomarker. Talanta 2021, 233, 122517. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Wu, J.; Chai, K.; Hu, X.; Hu, Y.; Shi, S.; Yao, T. A turn-on unlabeled colorimetric biosensor based on aptamer-AuNPs conjugates for amyloid-β oligomer detection. Talanta 2023, 260, 124649. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.A.; Park, S. AuNPs- Aβ-Ni-HRP sandwich assay: A new sensitive colorimetric method for the detection of Aβ1-40. Talanta 2022, 237, 122946. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.H.; Lee, D.H.; Nguyen, P.T.; Le, P.G.; Kim, M.I. Foldable paper microfluidic device based on single iron site-containing hydrogel nanozyme for efficient glucose biosensing. Chem. Eng. J. 2023, 454, 140541. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, K.; Su, Y.; Hu, S.; Liang, X.; Luo, Q.; Luo, H. Colorimetric and surface-enhanced Raman scattering dual-mode magnetic immunosensor for ultrasensitive detection of blood phosphorylated tau in Alzheimer’s disease. Biosens. Bioelectron. 2023, 222, 114935. [Google Scholar] [CrossRef] [PubMed]
- Le, H.T.N.; Kim, D.; Phan, L.M.T.; Cho, S. Ultrasensitive capacitance sensor to detect amyloid-beta1-40 in human serum using supramolecular recognition of β-CD/RGO/ITO micro-disk electrode. Talanta 2022, 237, 122907. [Google Scholar] [CrossRef]
- Ngoc Le, H.T.; Park, J.; Chinnadayyala, S.R.; Cho, S. Sensitive electrochemical detection of amyloid beta peptide in human serum using an interdigitated chain-shaped electrode. Biosens. Bioelectron. 2019, 144, 111694. [Google Scholar] [CrossRef]
- Zou, Y.; Chu, Z.; Guo, J.; Liu, S.; Ma, X.; Guo, J. Minimally invasive electrochemical continuous glucose monitoring sensors: Recent progress and perspective. Biosens. Bioelectron. 2023, 225, 115103. [Google Scholar] [CrossRef]
- Sinha, K.; Uddin, Z.; Kawsar, H.I.; Islam, S.; Deen, M.J.; Howlader, M.M.R. Analyzing chronic disease biomarkers using electrochemical sensors and artificial neural networks. TrAC Trends Anal. Chem. 2023, 158, 116861. [Google Scholar] [CrossRef]
- Tran, H.L.; Dang, V.D.; Dega, N.K.; Lu, S.-M.; Huang, Y.-F.; Doong, R.-a. Ultrasensitive detection of breast cancer cells with a lectin-based electrochemical sensor using N-doped graphene quantum dots as the sensing probe. Sens. Actuators B Chem. 2022, 368, 132233. [Google Scholar] [CrossRef]
- Cetinkaya, A.; Kaya, S.I.; Ozcelikay, G.; Atici, E.B.; Ozkan, S.A. A molecularly imprinted electrochemical sensor based on highly selective and an ultra-trace assay of anti-cancer drug axitinib in its dosage form and biological samples. Talanta 2021, 233, 122569. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhao, L.; Zhu, X.; Wei, X.; Zhu, M.; Ji, Q.; Luo, X.; Zhang, Y.; Zhou, Y.; Xu, M. Development of a novel ratiometric electrochemical sensor for monitoring β-galactosidase in Parkinson’s disease model mice. Biosens. Bioelectron. 2022, 210, 114301. [Google Scholar] [CrossRef] [PubMed]
- Kalinke, C.; De Oliveira, P.R.; Banks, C.E.; Janegitz, B.C.; Bonacin, J.A. 3D-printed immunosensor for the diagnosis of Parkinson’s disease. Sens. Actuators B Chem. 2023, 381, 133353. [Google Scholar] [CrossRef]
- Hideshima, S.; Wustoni, S.; Kobayashi, M.; Hayashi, H.; Kuroiwa, S.; Nakanishi, T.; Osaka, T. Effect of human serum on the electrical detection of amyloid-β fibrils in biological environments using azo-dye immobilized field effect transistor (FET) biosensor. Sens. Bio-Sens. Res. 2018, 17, 25–29. [Google Scholar] [CrossRef]
- Kwon, S.S.; Kim, D.; Yun, M.; Son, J.G.; Lee, S.H. The role of graphene patterning in field-effect transistor sensors to detect the tau protein for Alzheimer’s disease: Simplifying the immobilization process and improving the performance of graphene-based immunosensors. Biosens. Bioelectron. 2021, 192, 113519. [Google Scholar] [CrossRef]
- Wei, J.; Qiu, Z.; Yu, D.; Yin, Y.; Tang, Q.; Liao, X.; Zhang, G.; Liu, Z.; Gao, F. DNAzyme-driven tripedal DNA walker triggered hybridization chain reaction for label-free electrochemical detection of Alzheimer’s tau protein. Sens. Actuators B Chem. 2023, 384, 133656. [Google Scholar] [CrossRef]
- Sharma, P.K.; Kim, E.-S.; Mishra, S.; Ganbold, E.; Seong, R.-S.; Kim, Y.M.; Jahng, G.-H.; Rhee, H.Y.; Han, H.-S.; Kim, D.H.; et al. Ultrasensitive probeless capacitive biosensor for amyloid beta (Aβ1–42) detection in human plasma using interdigitated electrodes. Biosens. Bioelectron. 2022, 212, 114365. [Google Scholar] [CrossRef]
- Yoo, Y.K.; Kim, G.; Park, D.; Kim, J.; Kim, Y.; Yun Kim, H.; Yang, S.H.; Lee, J.H.; Hwang, K.S. Gold nanoparticles assisted sensitivity improvement of interdigitated microelectrodes biosensor for amyloid-β detection in plasma sample. Sens. Actuators B Chem. 2020, 308, 127710. [Google Scholar] [CrossRef]
- Park, D.; Kim, J.H.; Kim, H.J.; Lee, D.; Lee, D.S.; Yoon, D.S.; Hwang, K.S. Multiplexed femtomolar detection of Alzheimer’s disease biomarkers in biofluids using a reduced graphene oxide field-effect transistor. Biosens. Bioelectron. 2020, 167, 112505. [Google Scholar] [CrossRef]
- García-Chamé, M.-Á.; Gutiérrez-Sanz, Ó.; Ercan-Herbst, E.; Haustein, N.; Filipiak, M.S.; Ehrnhöfer, D.E.; Tarasov, A. A transistor-based label-free immunosensor for rapid detection of tau protein. Biosens. Bioelectron. 2020, 159, 112129. [Google Scholar] [CrossRef]
- Carneiro, P.; Morais, S.; do Carmo Pereira, M. Biosensors on the road to early diagnostic and surveillance of Alzheimer’s disease. Talanta 2020, 211, 120700. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Sun, S.; Chen, K.; Wang, Y.; Wang, H.; Meng, J.; Guo, M.; Zhang, X.-D.; Zhang, R. Emerging nanotechnology for Alzheimer’s disease: From detection to treatment. J. Control. Release 2023, 360, 392–417. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, J.; Khoshbin, Z.; Abnous, K.; Taghdisi, S.M.; Hosseinzadeh, H.; Danesh, N.M. Current progress in aptamer-based sensing tools for ultra-low level monitoring of Alzheimer’s disease biomarkers. Biosens. Bioelectron. 2022, 197, 113789. [Google Scholar] [CrossRef]
- Mathew, R.; Ajayan, J. Material processing, performance and reliability of MoS2 field effect transistor (FET) technology—A critical review. Mater. Sci. Semicond. Process. 2023, 160, 107397. [Google Scholar] [CrossRef]
- Schuck, A.; Kim, H.E.; Jung, K.-M.; Hasenkamp, W.; Kim, Y.-S. Monitoring the hemostasis process through the electrical characteristics of a graphene-based field-effect transistor. Biosens. Bioelectron. 2020, 157, 112167. [Google Scholar] [CrossRef] [PubMed]
- Veeralingam, S.; Badhulika, S. Surface functionalized β-Bi2O3 nanofibers based flexible, field-effect transistor-biosensor (BioFET) for rapid, label-free detection of serotonin in biological fluids. Sens. Actuators B Chem. 2020, 321, 128540. [Google Scholar] [CrossRef]
- Nekrasov, N.; Jaric, S.; Kireev, D.; Emelianov, A.V.; Orlov, A.V.; Gadjanski, I.; Nikitin, P.I.; Akinwande, D.; Bobrinetskiy, I. Real-time detection of ochratoxin A in wine through insight of aptamer conformation in conjunction with graphene field-effect transistor. Biosens. Bioelectron. 2022, 200, 113890. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y. Wafer-scale floating-gate field effect transistor sensor built on carbon nanotubes film for Ppb-level NO2 detection. Chem. Eng. J. 2023, 473, 145480. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, M.; He, J.; Zhang, Y.; Liang, Y.; Liu, H.; Zhang, Z. Aptamer-Functionalized Carbon Nanotube Field-Effect Transistor Biosensors for Alzheimer’s Disease Serum Biomarker Detection. ACS Sens. 2022, 7, 2075–2083. [Google Scholar] [CrossRef]
- Ozório, M.S.; Vieira, D.H.; Nogueira, G.L.; Martin, C.S.; Alves, N.; Constantino, C.J.L. Effect of the gate electrodes/water interface on the performance of ZnO-based water gate field-effect transistors. Mater. Sci. Semicond. Process. 2022, 151, 107045. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, J.; Wang, L.; Dong, B.; Mao, Y.; Qu, H.; Zheng, L. Selective detection of Pb2+ ions based on a graphene field-effect transistor gated by DNAzymes in binding mode. Biosens. Bioelectron. 2023, 237, 115549. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.-Y.; Li, B.-R.; Li, Y.-K. Silicon nanowire field-effect-transistor based biosensors: From sensitive to ultra-sensitive. Biosens. Bioelectron. 2014, 60, 101–111. [Google Scholar] [CrossRef]
- Dai, X.; Vo, R.; Hsu, H.-H.; Deng, P.; Zhang, Y.; Jiang, X. Modularized Field-Effect Transistor Biosensors. Nano Lett. 2019, 19, 6658–6664. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Massey, R.; Bebe, S.; Prakash, R. Aptamer-Enhanced Organic Electrolyte-Gated FET Biosensor for High-Specificity Detection of Cortisol. IEEE Sens. Lett. 2020, 4, 1–4. [Google Scholar] [CrossRef]
- Bergveld, P. The impact of MOSFET-based sensors. Sens. Actuators 1985, 8, 109–127. [Google Scholar] [CrossRef]
- Toumazou, C.; Georgiou, P. Piet Bergveld-40 years of ISFET technology: From neuronal sensing to DNA sequencing. Electron. Lett. 2011, 47, S7–S12. [Google Scholar] [CrossRef]
- Schöning, M.J.; Poghossian, A. Recent advances in biologically sensitive field-effect transistors (BioFETs). Analyst 2002, 127, 1137–1151. [Google Scholar] [CrossRef]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.-H.; Choi, M.; Ku, K.B.; Lee, C.-S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef]
- Shin, C.J.; Seo, S.E.; Nam, Y.; Kim, K.H.; Kim, L.; Kim, J.; Ryu, E.; Hwang, J.Y.; Kim, G.-J.; Jung, M.-W.; et al. Real-time monitoring of cyanobacterial harmful algal blooms by graphene field-effect transistor. Chem. Eng. J. 2023, 459, 141419. [Google Scholar] [CrossRef]
- Yang, J.C.; Lim, S.J.; Cho, C.H.; Hazarika, D.; Park, J.P.; Park, J. Determination of tumor necrosis factor-α in serum using extended-gate field-effect transistor-based chemosensors with molecularly imprinted polymer-coated gold dendrites. Sens. Actuators B Chem. 2023, 390, 133982. [Google Scholar] [CrossRef]
- Bartold, K.; Iskierko, Z.; Borowicz, P.; Noworyta, K.; Lin, C.-Y.; Kalecki, J.; Sharma, P.S.; Lin, H.-Y.; Kutner, W. Molecularly imprinted polymer-based extended-gate field-effect transistor (EG-FET) chemosensor for selective determination of matrix metalloproteinase-1 (MMP-1) protein. Biosens. Bioelectron. 2022, 208, 114203. [Google Scholar] [CrossRef]
- Huang, C.; Hao, Z.; Wang, Z.; Zhao, X.; Wang, H.; Li, F.; Liu, S.; Pan, Y. A fully integrated graphene-polymer field-effect transistor biosensing device for on-site detection of glucose in human urine. Mater. Today Chem. 2022, 23, 100635. [Google Scholar] [CrossRef]
- Shen, Y.; Chai, S.; Zhang, Q.; Zhang, M.; Mao, X.; Wei, L.; Zhou, F.; Sun, R.; Liu, C. PVF composite conductive nanofibers-based organic electrochemical transistors for lactate detection in human sweat. Chem. Eng. J. 2023, 475, 146008. [Google Scholar] [CrossRef]
- Xu, Q.; Zong, B.; Li, Q.; Fang, X.; Mao, S.; Ostrikov, K. H2S sensing under various humidity conditions with Ag nanoparticle functionalized Ti3C2Tx MXene field-effect transistors. J. Hazard. Mater. 2022, 424, 127492. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Tripathy, N.; Hahn, Y.-B. High-performance cholesterol sensor based on the solution-gated field effect transistor fabricated with ZnO nanorods. Biosens. Bioelectron. 2013, 45, 281–286. [Google Scholar] [CrossRef]
- Bian, L.; Wang, Z.; White, D.L.; Star, A. Machine learning-assisted calibration of Hg2+ sensors based on carbon nanotube field-effect transistors. Biosens. Bioelectron. 2021, 180, 113085. [Google Scholar] [CrossRef]
- Oh, J.; Yoo, G.; Chang, Y.W.; Kim, H.J.; Jose, J.; Kim, E.; Pyun, J.-C.; Yoo, K.-H. A carbon nanotube metal semiconductor field effect transistor-based biosensor for detection of amyloid-beta in human serum. Biosens. Bioelectron. 2013, 50, 345–350. [Google Scholar] [CrossRef]
- Tseng, A.C.; Ito, K.; Lynall, D.; Savelyev, I.G.; Blumin, M.; Wang, S.; Ruda, H.E.; Sakata, T. Ion sensitivity from current hysteresis in InAs nanowire field-effect transistors functionalized with ionophore-doped fluorosilicone membranes. Sens. Actuators B Chem. 2021, 336, 129704. [Google Scholar] [CrossRef]
- Wei, J.; Liu, Z.; Zhang, Z.; Lan, K.; Wang, Y.; Chen, R.; Qin, G. Suspended CNTs/MoS2 heterostructure field effect transistor for high performance biosensor and its application for serum PSA detection. Sens. Actuators B Chem. 2023, 381, 133417. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, D.; Xu, Y.; Yin, Z.; Dou, W.; Habiba, U.E.; Pan, C.; Zhang, Z.; Mou, H.; Deng, H.; et al. DNA-based functionalization of two-dimensional MoS2 FET biosensor for ultrasensitive detection of PSA. Appl. Surf. Sci. 2021, 548, 149169. [Google Scholar] [CrossRef]
- Chen, X.; Hao, S.; Zong, B.; Liu, C.; Mao, S. Ultraselective antibiotic sensing with complementary strand DNA assisted aptamer/MoS2 field-effect transistors. Biosens. Bioelectron. 2019, 145, 111711. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, M.; Zhang, Y.; Ji, H.; Gao, J.; Song, S.; Sun, J.; Liu, H.; Zhang, Y.; Han, L. Ultrasensitive Antibiotic Perceiving Based on Aptamer-Functionalized Ultraclean Graphene Field-Effect Transistor Biosensor. Anal. Chem. 2022, 94, 14785–14793. [Google Scholar] [CrossRef]
- Samota, S.; Rani, R.; Chakraverty, S.; Kaushik, A. Biosensors for simplistic detection of pathogenic bacteria: A review with special focus on field-effect transistors. Mater. Sci. Semicond. Process. 2022, 141, 106404. [Google Scholar] [CrossRef]
- Sun, Q.; Ma, C.; Li, W.; Li, X.; Sakamoto, K.; Liu, X.; Okamoto, A.; Minari, T. Fully Printed Low-Voltage Field-Effect Transistor Biosensor Array for One-Drop Detection of Shewanella onedensis MR-1 Bacteria. ACS Appl. Electron. Mater. 2023, 5, 2558–2565. [Google Scholar] [CrossRef]
- Shariati, M.; Vaezjalali, M.; Sadeghi, M. Ultrasensitive and easily reproducible biosensor based on novel doped MoS2 nanowires field-effect transistor in label-free approach for detection of hepatitis B virus in blood serum. Anal. Chim. Acta 2021, 1156, 338360. [Google Scholar] [CrossRef] [PubMed]
- Fathi-Hafshejani, P.; Azam, N.; Wang, L.; Kuroda, M.A.; Hamilton, M.C.; Hasim, S.; Mahjouri-Samani, M. Two-Dimensional-Material-Based Field-Effect Transistor Biosensor for Detecting COVID-19 Virus (SARS-CoV-2). ACS Nano 2021, 15, 11461–11469. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, Y.; Lee, T.; Ahn, J.-H. Aptamer-Based Field-Effect Transistor for Detection of Avian Influenza Virus in Chicken Serum. Anal. Chem. 2020, 92, 5524–5531. [Google Scholar] [CrossRef]
- Rashid, R.B.; Ji, X.; Rivnay, J. Organic electrochemical transistors in bioelectronic circuits. Biosens. Bioelectron. 2021, 190, 113461. [Google Scholar] [CrossRef]
- Ratnesh, R.K.; Goel, A.; Kaushik, G.; Garg, H.; Chandan; Singh, M.; Prasad, B. Advancement and challenges in MOSFET scaling. Mater. Sci. Semicond. Process. 2021, 134, 106002. [Google Scholar] [CrossRef]
- Ciou, S.-H.; Hsieh, A.-H.; Lin, Y.-X.; Sei, J.-L.; Govindasamy, M.; Kuo, C.-F.; Huang, C.-H. Sensitive label-free detection of the biomarker phosphorylated tau−217 protein in Alzheimer’s disease using a graphene-based solution-gated field effect transistor. Biosens. Bioelectron. 2023, 228, 115174. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, Y.; Zhou, Y.; Liu, Y.; Chen, S.; Sun, W.; Zhang, Z.; Guo, J.; Yang, C.; Li, Z.; et al. Unamplified system for sensitive and typing detection of ASFV by the cascade platform that CRISPR-Cas12a combined with graphene field-effect transistor. Biosens. Bioelectron. 2023, 240, 115637. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Kim, S.; Lee, D.; Chung, R.B.K. High-performance tin oxide field-effect transistors deposited by thermal atomic layer deposition. Mater. Today Commun. 2023, 37, 107064. [Google Scholar] [CrossRef]
- Chen, X.; Wan, J.; Wu, H.; Liu, C. ZnO bilayer thin film transistors using H2O and O3 as oxidants by atomic layer deposition. Acta Mater. 2020, 185, 204–210. [Google Scholar] [CrossRef]
- Sanda, S.; Nakamichi, R.; Nagase, T.; Kobayashi, T.; Takimiya, K.; Sadamitsu, Y.; Naito, H. Effect of non-chlorinated solvents on the enhancement of field-effect mobility in dioctylbenzothienobenzothiophene-based top-gate organic transistors processed by spin coating. Org. Electron. 2019, 69, 181–189. [Google Scholar] [CrossRef]
- Nehra, A.; Pal Singh, K. Current trends in nanomaterial embedded field effect transistor-based biosensor. Biosens. Bioelectron. 2015, 74, 731–743. [Google Scholar] [CrossRef]
- Wang, W.; Lu, J.; Wan, D.; Zeng, X.; Lu, J.; Xu, T.; Chen, C.; Zhang, T. Improved performance in MoS2 homogeneous junction field effect transistors by optimizing electrodes contact. Mater. Sci. Eng. B 2023, 290, 116348. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, Z.; Lan, K.; Wang, Z.; Qin, G.; Chen, R. Highly sensitive detection of multiple proteins from single cells by MoS2-FET biosensors. Talanta 2022, 236, 122839. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, H.; Zhuo, J.; Zhu, Z.; Papakonstantinou, P.; Lubarsky, G.; Lin, J.; Li, M. Biosensor Based on Ultrasmall MoS2 Nanoparticles for Electrochemical Detection of H2O2 Released by Cells at the Nanomolar Level. Anal. Chem. 2013, 85, 10289–10295. [Google Scholar] [CrossRef]
- He, W.; Huang, Y.; Wu, J. Enzyme-Free Glucose Biosensors Based on MoS2 Nanocomposites. Nanoscale Res. Lett. 2020, 15, 60. [Google Scholar] [CrossRef]
- Sarkar, D.; Liu, W.; Xie, X.; Anselmo, A.C.; Mitragotri, S.; Banerjee, K. MoS2 Field-Effect Transistor for Next-Generation Label-Free Biosensors. ACS Nano 2014, 8, 3992–4003. [Google Scholar] [CrossRef] [PubMed]
- Le, P.G.; Wu, Q.; Kong, D.Y.; Ge, J.; Il Kim, M. Tailoring Nanostructured Supports to Achieve High Performance in Enzymatic Biofuel Cells. ACS Appl. Energy Mater. 2022, 5, 13113–13127. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Eigler, S. Graphene Oxide: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Xu, S.; Wang, T.; Liu, G.; Cao, Z.; Frank, L.A.; Jiang, S.; Zhang, C.; Li, Z.; Krasitskaya, V.V.; Li, Q.; et al. Analysis of interactions between proteins and small-molecule drugs by a biosensor based on a graphene field-effect transistor. Sens. Actuators B Chem. 2021, 326, 128991. [Google Scholar] [CrossRef]
- Chen, S.; Sun, Y.; Xia, Y.; Lv, K.; Man, B.; Yang, C. Donor effect dominated molybdenum disulfide/graphene nanostructure-based field-effect transistor for ultrasensitive DNA detection. Biosens. Bioelectron. 2020, 156, 112128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhou, T.; Chen, M.; Feng, H.; Yuan, R.; Zhong, C.a.; Yan, W.; Tian, Y.; Wu, X.; Chu, W.; et al. High-purity pyrrole-type FeN4 sites as a superior oxygen reduction electrocatalyst. Energy Environ. Sci. 2020, 13, 111–118. [Google Scholar] [CrossRef]
- Duan, Z.; Henkelman, G. Surface Charge and Electrostatic Spin Crossover Effects in CoN4 Electrocatalysts. ACS Catal. 2020, 10, 12148–12155. [Google Scholar] [CrossRef]
- Ulman, A. Formation and Structure of Self-Assembled Monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef]
- Gao, J.; Wang, C.; Wang, C.; Chu, Y.; Wang, S.; Sun, M.Y.; Ji, H.; Gao, Y.; Wang, Y.; Han, Y.; et al. Poly-l-Lysine-Modified Graphene Field-Effect Transistor Biosensors for Ultrasensitive Breast Cancer miRNAs and SARS-CoV-2 RNA Detection. Anal. Chem. 2022, 94, 1626–1636. [Google Scholar] [CrossRef]
- Le, P.G.; Kim, M.I. Research Progress and Prospects of Nanozyme-Based Glucose Biofuel Cells. Nanomaterials 2021, 11, 2116. [Google Scholar] [CrossRef]
- Chen, L.; Li, G.; Yang, A.; Wu, J.; Yan, F.; Ju, H. A DNA-functionalized graphene field-effect transistor for quantitation of vascular endothelial growth factor. Sens. Actuators B Chem. 2022, 351, 130964. [Google Scholar] [CrossRef]
- Bagherzadeh-Nobari, S.; Kalantarinejad, R. Real-time label-free detection of DNA hybridization using a functionalized graphene field effect transistor: A theoretical study. J. Nanoparticle Res. 2021, 23, 185. [Google Scholar] [CrossRef]
- Lu, H.-W.; Kane, A.A.; Parkinson, J.; Gao, Y.; Hajian, R.; Heltzen, M.; Goldsmith, B.; Aran, K. The promise of graphene-based transistors for democratizing multiomics studies. Biosens. Bioelectron. 2022, 195, 113605. [Google Scholar] [CrossRef]
- Palazzo, G.; De Tullio, D.; Magliulo, M.; Mallardi, A.; Intranuovo, F.; Mulla, M.Y.; Favia, P.; Vikholm-Lundin, I.; Torsi, L. Detection Beyond Debye’s Length with an Electrolyte-Gated Organic Field-Effect Transistor. Adv. Mater. 2015, 27, 911–916. [Google Scholar] [CrossRef]
- Liang, Y.; Xiao, M.; Wu, D.; Lin, Y.; Liu, L.; He, J.; Zhang, G.; Peng, L.-M.; Zhang, Z. Wafer-Scale Uniform Carbon Nanotube Transistors for Ultrasensitive and Label-Free Detection of Disease Biomarkers. ACS Nano 2020, 14, 8866–8874. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sun, Y.; Fan, X.; Xu, Y.; Chen, S.; Zhang, X.; Man, B.; Yang, C.; Du, J. Review on two-dimensional material-based field-effect transistor biosensors: Accomplishments, mechanisms, and perspectives. J. Nanobiotechnol. 2023, 21, 144. [Google Scholar] [CrossRef] [PubMed]
- Salehirozveh, M.; Dehghani, P.; Zimmermann, M.; Roy, V.A.L.; Heidari, H. Graphene Field Effect Transistor Biosensors Based on Aptamer for Amyloid-β Detection. IEEE Sens. J. 2020, 20, 12488–12494. [Google Scholar] [CrossRef]
- Wustoni, S.; Wang, S.; Alvarez, J.R.; Hidalgo, T.C.; Nunes, S.P.; Inal, S. An organic electrochemical transistor integrated with a molecularly selective isoporous membrane for amyloid-β detection. Biosens. Bioelectron. 2019, 143, 111561. [Google Scholar] [CrossRef]
- Balderston, S.; Taulbee, J.J.; Celaya, E.; Fung, K.; Jiao, A.; Smith, K.; Hajian, R.; Gasiunas, G.; Kutanovas, S.; Kim, D.; et al. Discrimination of single-point mutations in unamplified genomic DNA via Cas9 immobilized on a graphene field-effect transistor. Nat. Biomed. Eng. 2021, 5, 713–725. [Google Scholar] [CrossRef]
- Hajian, R.; Balderston, S.; Tran, T.; deBoer, T.; Etienne, J.; Sandhu, M.; Wauford, N.A.; Chung, J.-Y.; Nokes, J.; Athaiya, M.; et al. Detection of unamplified target genes via CRISPR–Cas9 immobilized on a graphene field-effect transistor. Nat. Biomed. Eng. 2019, 3, 427–437. [Google Scholar] [CrossRef]
- Singh, A.; Khatun, S.; Nath Gupta, A. Simultaneous Detection of Tyrosine and Structure-Specific Intrinsic Fluorescence in the Fibrillation of Alzheimer’s Associated Peptides. ChemPhysChem 2020, 21, 2585–2598. [Google Scholar] [CrossRef]
- Suprun, E.V.; Khmeleva, S.A.; Radko, S.P.; Kozin, S.A.; Archakov, A.I.; Shumyantseva, V.V. Direct electrochemical oxidation of amyloid-β peptides via tyrosine, histidine, and methionine residues. Electrochem. Commun. 2016, 65, 53–56. [Google Scholar] [CrossRef]
- Jamerlan, A.; An, S.S.A.; Hulme, J. Advances in amyloid beta oligomer detection applications in Alzheimer’s disease. TrAC Trends Anal. Chem. 2020, 129, 115919. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, A.; Tang, X.; Qin, J. Electrochemical sensitive detection of amyloid-β oligomer harnessing cellular prion protein on AuNPs embedded poly (pyrrole-3-carboxylic acid) matrix. Mater. Today Adv. 2022, 14, 100250. [Google Scholar] [CrossRef]
- Qin, J.; Park, J.S.; Jo, D.G.; Cho, M.; Lee, Y. Curcumin-based electrochemical sensor of amyloid-β oligomer for the early detection of Alzheimer’s disease. Sens. Actuators B Chem. 2018, 273, 1593–1599. [Google Scholar] [CrossRef]
- Kim, K.; Kim, M.-J.; Kim, D.W.; Kim, S.Y.; Park, S.; Park, C.B. Clinically accurate diagnosis of Alzheimer’s disease via multiplexed sensing of core biomarkers in human plasma. Nat. Commun. 2020, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Fenoy, G.E.; Marmisollé, W.A.; Azzaroni, O.; Knoll, W. Acetylcholine biosensor based on the electrochemical functionalization of graphene field-effect transistors. Biosens. Bioelectron. 2020, 148, 111796. [Google Scholar] [CrossRef]
- Li, J.; Ni, W.; Jin, D.; Yu, Y.; Xiao, M.-M.; Zhang, Z.-Y.; Zhang, G.-J. Nanosensor-Driven Detection of Neuron-Derived Exosomal Aβ42 with Graphene Electrolyte-Gated Transistor for Alzheimer’s Disease Diagnosis. Anal. Chem. 2023, 95, 5719–5728. [Google Scholar] [CrossRef]
- Arano-Martinez, J.A.; Martínez-González, C.L.; Salazar, M.I.; Torres-Torres, C. A Framework for Biosensors Assisted by Multiphoton Effects and Machine Learning. Biosensors 2022, 12, 710. [Google Scholar] [CrossRef]






| No. | Electrode Materials | Biofluids | Analyte | Linear Range | LOD | Ref. | |
|---|---|---|---|---|---|---|---|
| Source, Drain, Gate | Channel Layer | ||||||
| 1 | Commercialized n-type FET chip | SiO2/APTES/GA/CR | HSA | Aβ1-42 | 10 pM–100 µM | NA * | [28] |
| 2 | SiO2/APTES/GA/Aβ1-42 Ab | ||||||
| 3 | Ti/Au, Ti/Au, Ag/AgCl | Au/thiolate PEG (12 kDa)-COOH-EG8-thiol/EDC-NHS/Tau Ab/BSA | CSF | Tau | 1 pM–10 nM | <1 pM | [34] |
| Ti/Au, Ti/Au, Ag/AgCl | Au/thiolate PEG (20 kDa)-COOH-EG8-thiol/EDC-NHS/Tau Ab/BSA | <10 pM | |||||
| 4 | Ti/Pt, Ti/Pt, Ag/AgCl | G/PSE/Tau Ab/BSA | PBS, HS, and HP | Tau | 10 fg/mL–1 ng/mL | NA | [29] |
| 5 | Ti/Au, Ti/Au, Ag/AgCl | Si/SiO2/rGO/PBASE/Tau Ab | PBS | Aβ1-42 | 1 pg/mL–100 ng/mL | NA | [33] |
| t-Tau | 100 fg/mL–1 ng/mL | NA | |||||
| CSF, HP | Aβ1-42 | 100 fg/mL–100 ng/mL | 222 fM | ||||
| t-Tau | 100 fg/mL–100 ng/mL | 21.8 fM | |||||
| 6 | Au/Cr, Au/Cr, Organic semiconductor | Kapton/Au/PFBT/α-synuclein Ab | PBS | α-synuclein | 0.25 pM to 25 nM | 0.25 pM | [9] |
| 7 | Ti/Au, Ti/Au, Ag/AgCl | rGO/PBASE/AchE | PBS | Acetylcholine | 1 µM to 10 mM | 13.9 mV/dec | [8] |
| 8 | Cr/Au, Cr/Au, Cr/Au | Si/Al2O3/BG/p-tau Ab | PBS | p-tau217 | 10 fg/mL to 100 pg/mL | 18.6 mV/dec | [66] |
| 9 | HSA | 16.7 mV/dec | |||||
| 10 | NA | SiO2/PDOT: PSS/CR | PBS | Aβ1-42 | 2.21 pM–221 nM | 216 μA/dec | [89] |
| 11 | Pb, Pb, Ag/AgCl | SiO2 NW/GPTES/ssDNA aptamer | PBS | Aβ1-40 | 0.1 pg/mL–10 µg/mL | 20 fM | [92] |
| 12 | Au, Au, Ag wire | rGO/Au/NHS-EDC/Aβ1-42 Ab | PBS | Aβ1-42NDE–Aβ1-42 | 1.48–148 pg/mL | 447 ag/mL | [94] |
| 13 | Ti/Pd/Au, Ti/Pd/Au, Y2O3 | CNT/Au/DNA aptamer | Serum | Aβ1-42 | 1 fM to 10 pM | 45 aM | [40] |
| 14 | Aβ1-40 | 55 aM | |||||
| 15 | Au/Al2O3, Au/Al2O3, top gate | Si/SiO2/APTES/rGO/RNA aptamer/BSA | PBS | Aβ1-42 | 1 ng/mL–1 pg/mL | NA | [93] |
| 16 | Cr/Au, Cr/Au, | CNT/sulfo-NHE/Ab/BSA | PBS | Aβ1-42 | 100 to 106 fM | 2.13 fM | [90] |
| Aβ1-40 | 2.20 fM | ||||||
| t-tau | 2.45 fM | ||||||
| p-tau181 | 2.72 fM | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, P.G.; Choi, S.H.; Cho, S. Alzheimer’s Disease Biomarker Detection Using Field Effect Transistor-Based Biosensor. Biosensors 2023, 13, 987. https://doi.org/10.3390/bios13110987
Le PG, Choi SH, Cho S. Alzheimer’s Disease Biomarker Detection Using Field Effect Transistor-Based Biosensor. Biosensors. 2023; 13(11):987. https://doi.org/10.3390/bios13110987
Chicago/Turabian StyleLe, Phan Gia, Seong Hye Choi, and Sungbo Cho. 2023. "Alzheimer’s Disease Biomarker Detection Using Field Effect Transistor-Based Biosensor" Biosensors 13, no. 11: 987. https://doi.org/10.3390/bios13110987
APA StyleLe, P. G., Choi, S. H., & Cho, S. (2023). Alzheimer’s Disease Biomarker Detection Using Field Effect Transistor-Based Biosensor. Biosensors, 13(11), 987. https://doi.org/10.3390/bios13110987