Impedimetric DNA Sensor Based on Electropolymerized N-Phenylaminophenothiazine and Thiacalix[4]arene Tetraacids for Doxorubicin Determination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus
2.3. Electrode Modification
2.4. Doxoruibcin Determination
3. Results
3.1. Polymerization of PhTz and Cyclic Voltammetry of the Surface Layer Obtained
3.2. AFM Measurements
3.3. EIS Measurements
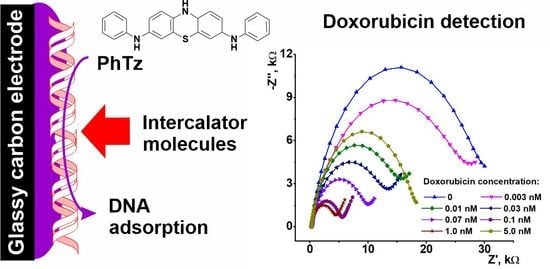
3.4. Doxorubicin Determination
3.4.1. Measurement Conditions
3.4.2. Measurement Precision and Lifetime
3.4.3. Selectivity and Real Sample Assay
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tavares, A.P.M.; Truta, L.A.A.N.A.; Moreira, F.T.C.; Carneiro, L.P.T.; Sales, M.G.F. Self-powered and self-signalled autonomous electrochemical biosensor applied to cancinoembryonic antigen determination. Biosens. Bioelectron. 2019, 140, 111320. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Xu, T.T.; Fang, L.Y.; Chen, H.; Huang, Y.Y.; Zhang, H.Y.; Miao, Z.Y.; Mao, C.; Chi, B.; Xu, H. A blood compatible, high-efficient sensor for detection of Cr(VI) in whole blood. Sens. Actuators B 2021, 329, 129219. [Google Scholar] [CrossRef]
- Joice, E.K.; Rison, S.; Akshaya, K.B.; Varghese, A. Platinum decorated polythiophene modified stainless steel for electrocatalytic oxidation of benzyl alcohol. J. Appl. Electrochem. 2019, 49, 937–947. [Google Scholar] [CrossRef]
- Schneider, S.; Fuser, M.; Bolte, M.; Terfort, A. Self-assembled monolayers of aromatic pyrrole derivatives: Electropolymerization and electrocopolymerization with pyrrole. Electrochim. Acta 2017, 246, 853–863. [Google Scholar] [CrossRef]
- Li, S.P.; Zhou, J.Y.; Noroozifar, M.; Kerman, K. Gold-platinum core-shell nanoparticles with thiolated polyaniline and multi-walled carbon nanotubes for the simultaneous voltammetric determination of six drug molecules. Chemosensors 2021, 9, 24. [Google Scholar] [CrossRef]
- Guven, N.; Sultanova, H.; Ozer, B.; Yucel, B.; Camurlu, P. Tuning of electrochromic properties of electrogenerated polythiophenes through Ru(II) complex tethering and backbone derivatization. Electrochim. Acta 2020, 329, 135134. [Google Scholar] [CrossRef]
- Tkach, V.V.; de Paiva Martins, J.I.M.; Ivanushko, Y.G.; Yagodynets’, P.I. Dye electropolymerization for electrochemical analysis. a brief review. Biointerface Res. Appl. Chem. 2022, 12, 4028–4047. [Google Scholar] [CrossRef]
- Dalkiran, B.; Brett, C.M.A. Polyphenazine and polytriphenylmethane redox polymer/nanomaterial–based electrochemical sensors and biosensors: A review. Microchim. Acta 2021, 188, 178. [Google Scholar] [CrossRef]
- Choi, E.J.; Drago, N.P.; Humphrey, N.J.; Van Houten, J.; Ahn, J.; Lee, J.; Kim, I.D.; Ogata, A.F.; Penner, R.M. lectrodeposition-enabled, electrically-transduced sensors and biosensors. Mater. Today 2023, 62, 129–150. [Google Scholar] [CrossRef]
- Yang, D.; Wang, J.; Cao, Y.; Tong, X.; Hua, T.; Qin, R.; Shao, Y. Polyaniline-based biological and chemical sensors: Sensing mechanism, configuration design, and perspective. ACS Appl. Electron. Mater. 2023, 5, 593–611. [Google Scholar] [CrossRef]
- Majeed, A.H.; Mohammed, L.A.; Hammoodi, O.G.; Sehgal, S.; Alheety, M.A.; Saxena, K.K.; Dadoosh, S.A.; Mohammed, I.K.; Jasim, M.M.; Salmaan, N.U. A Review on polyaniline: Synthesis, properties, nanocomposites, and electrochemical applications. Int. J. Polym. Sci. 2022, 2022, 9047554. [Google Scholar] [CrossRef]
- Cosnier, S. Affinity biosensors based on electropolymerized films. Electroanalysis 2005, 17, 1701–1715. [Google Scholar] [CrossRef]
- Evtugyn, G.; Hianik, T. Electrochemical DNA sensors and aptasensors based on electropolymerized materials and polyelectrolyte complexes. TrAC Trends Anal. Chem. 2016, 79, 168–178. [Google Scholar] [CrossRef]
- Martínez-Rojas, F.; Castañeda, E.; Armijo, F. Conducting polymer applied in a label-free electrochemical immunosensor for the detection prostate-specific antigen using its redox response as an analytical signal. J. Electroanal. Chem. 2021, 880, 114877. [Google Scholar] [CrossRef]
- Ghoorchian, A.; Madrakian, T.; Afkhami, A.; Bagheri, H. Spectroelectrochemical and electrochromic behavior of poly(methylene blue) and poly(thionine)-modified multi-walled carbon nanotubes. J. Solid State Electrochem. 2021, 25, 1217–1229. [Google Scholar] [CrossRef]
- Mokhtari, Z.; Khajehsharifi, H.; Hashemnia, S.; Solati, Z.; Azimpanah, R.; Shahrokhian, S. Evaluation of molecular imprinted polymerized methylene blue/aptamer as a novel hybrid receptor for Cardiac Troponin I (cTnI) detection at glassy carbon electrodes modified with new biosynthesized ZnO NPs. Sens. Actuators B 2020, 320, 128316. [Google Scholar] [CrossRef]
- Pandey, I.; Bairagi, P.K.; Verma, N. Electrochemically grown polymethylene blue nanofilm on copper-carbon nanofiber nanocomposite: An electrochemical sensor for creatinine. Sens. Actuators B. 2018, 277, 562–570. [Google Scholar] [CrossRef]
- Zhu, L.; Wei, Z.; Wang, J.; Zhong, J. An electrochemical biosensor based on NiO nanoflowers/polymethylene blue composite for non-enzymatic glucose detection. J. Electrochem. Soc. 2020, 167, 146512. [Google Scholar] [CrossRef]
- Tsuruoka, N.; Soto, S.S.; Ben Tahar, A.; Zebda, A.; Tsujimura, S. Mediated electrochemical oxidation of glucose via poly(methylene green) grafted on the carbon surface catalyzed by flavin adenine dinucleotide-dependent glucose dehydrogenase. Colloids Surf. B. 2020, 192, 111065. [Google Scholar] [CrossRef]
- Porfireva, A.V.; Goida, A.I.; Rogov, A.M.; Evtugyn, G.A. Impedimetric DNA sensor based on poly(proflavine) for determination of anthracycline drugs. Electroanalysis 2020, 32, 827–834. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, L.M.; Yang, S.Q. Amplification of bioelectrocatalytic signalling based on silver nanoparticles and DNA-derived horseradish peroxidase biosensors. Microchim. Acta 2008, 160, 357–365. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, C.; Su, X.; Zhang, W.; Zou, X. Signal on-off ratiometric electrochemical sensor based on semi-complementary aptamer couple for sensitive cadmium detection in mussel. Sens. Actuators B 2021, 346, 130506. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, M.; Shan, Y.; Jin, Y.; Gong, M.; Wang, X. Nano polythionine-based electrochemiluminescence biosensor for detection of the p16INK4a gene using RuAg@AuNPs core-shell nanocomposites as DNA labels. J. Lumin. 2018, 201, 135–142. [Google Scholar] [CrossRef]
- Aguilar-Ortíz, E.; Zaragoza-Galán, G.; Solladié, N.; Rein, R.; Aguilar-Martínez, M.; Macías-Ruvalcaba, N.; Rivera, E. Preparation and characterization of novel polythiophenes bearing oligo (ethylene glycol) spacers and porphyrin units: Optical and electrochemical properties. Synth. Met. 2012, 162, 1000–1009. [Google Scholar] [CrossRef]
- Kuzin, Y.I.; Padnya, P.L.; Stoikov, I.I.; Gorbatchuk, V.V.; Stoikov, D.I.; Khadieva, A.I.; Evtugyn, G.A. Electrochemical behavior of the monomeric and polymeric forms of N-phenyl-3-(phenylimino)-3H-phenothiazin-7-amine. Electrochim. Acta 2020, 345, 136195. [Google Scholar] [CrossRef]
- Kulikova, T.; Padnya, P.; Shiabiev, I.; Rogov, A.; Stoikov, I.; Evtugyn, G. Electrochemical sensing of interactions between DNA and charged macrocycles. Chemosensors 2021, 9, 347. [Google Scholar] [CrossRef]
- Kuzin, Y.I.; Gorbatchuk, V.V.; Rogov, A.M.; Stoikov, I.I.; Evtugyn, G.A. Electrochemical properties of multilayered coatings implementing thiacalix[4]arenes with oligolactic fragments and DNA. Electroanalysis 2020, 32, 715–723. [Google Scholar] [CrossRef]
- Gorbatchuk, V.V.; Porfireva, A.V.; Stepanova, V.B.; Kuzin, Y.I.; Evtugyn, V.G.; Shamagsumova, R.V.; Stoikov, I.I.; Evtugyn, G.A. Co-polymers of oligolactic acid and tetrasubstituted thiacalix[4]arenes as a new material for electrochemical sensor development. Sens. Actuators B 2017, 246, 136–145. [Google Scholar] [CrossRef]
- Khadieva, A.; Gorbachuk, V.; Shurpik, D.; Stoikov, I. Synthesis of tris-pillar[5]arene and its association with phenothiazine dye: Colorimetric recognition of anions. Molecules 2019, 24, 1807. [Google Scholar] [CrossRef]
- Stoikov, I.I.; Smolentsev, V.A.; Antipin, I.S.; Habicher, W.; Gruner, M.; Konovalov, A.I. Array of fluorescent chemosensors for the molecular recognition of halide anions on the basis of the stereoisomers of thiacalix[4]arene tetranaphthylamides. Mendeleev Commun. 2006, 16, 294–297. [Google Scholar] [CrossRef]
- Hongpaisan, J.; Roomans, G.M. Retaining ionic concentrations during in vitro storage of tissue for microanalytical studies. J. Microsc. 1999, 193, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Kulikova, T.; Porfireva, A.; Evtugyn, G.; Hianik, T. Electrochemical DNA sensors with layered polyaniline-DNA coating for detection of specific DNA interactions. Sensors 2019, 19, 469. [Google Scholar] [CrossRef] [PubMed]
- Arcamone, F.; Cassinelli, G.; Fantini, G.; Grein, A.; Orezzi, P.; Pol, C.; Spalla, C. Adriamycin, 14-hydroxydaunomycin, a new antitumor antibiotic from S. peucetius var. caesius. Biotechnol. Bioeng. 1969, 11, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Van der Zanden, S.Y.; Qiao, X.; Neefjes, J. New insights into the activities and toxicities of the old anticancer drug doxorubicin. FEBS J. 2021, 288, 6095–6111. [Google Scholar] [CrossRef]
- Liao, L.B.; Zhou, H.Y.; Xiao, X.M. Spectroscopic and viscosity study of doxorubicin interaction with DNA. J. Mol. Struct. 2005, 749, 108–113. [Google Scholar] [CrossRef]
- Stratigou, I.-C.; Tsiasioti, A.; Tzanavaras, P.D.; Markopoulou, C.K.; Fytianos, K.; Zacharis, C.K. Homogeneous liquid liquid extraction using salt as mass separating agent for the ultra high pressure liquid chromatographic determination of doxorubicin in human urine. Microchem. J. 2020, 158, 105260. [Google Scholar] [CrossRef]
- Rahmani, F.; Hosseini, M.-R.M.; Es-haghi, A.; Mollahosseini, A. A 96-Monolithic inorganic hollow fiber array as a new geometry for high throughput solid-phase microextraction of doxorubicin in water and human urine samples coupled with liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2020, 1627, 461413. [Google Scholar] [CrossRef]
- Liu, Y.; Danielsson, B. Rapid high throughput assay for fluorimetric detection of doxorubicin—Application of nucleic acid–dye bioprobe. Anal. Chim. Acta 2007, 587, 47–51. [Google Scholar] [CrossRef]
- Anderson, A.B.; Ciriacks, C.M.; Fuller, K.M.; Arriaga, E.A. Distribution of zeptomole-abundant doxorubicin metabolites in subcellular fractions by capillary electrophoresis with laser-induced fluorescence detection. Anal. Chem. 2003, 75, 8–15. [Google Scholar] [CrossRef]
- El-Maghrabey, M.; Kishikawa, N.; Kamimura, S.; Ohyama, K.; Kuroda, N. Design of a dual functionalized chemiluminescence ultrasensitive probe for quinones based on their redox cycle. Application to the determination of doxorubicin in lyophilized powder and human serum. Sens. Actuators B 2021, 329, 129226. [Google Scholar] [CrossRef]
- Suprun, E.V.; Kutdusova, G.R.; Khmeleva, S.A.; Radko, S.P. Towards deeper understanding of DNA electrochemical oxidation on carbon electrodes. Electrochem. Commun. 2021, 124, 106947. [Google Scholar] [CrossRef]
- Goida, A.; Kuzin, Y.; Evtugyn, V.; Porfireva, A.; Evtugyn, G.; Hianik, T. Electrochemical sensing of idarubicin—DNA interaction using electropolymerized Azure B and Methylene blue mediation. Chemosensors 2022, 10, 33. [Google Scholar] [CrossRef]
- Liu, J.; Bo, X.; Zhou, M.; Guo, L. A nanocomposite prepared from metal-free mesoporous carbon nanospheres and graphene oxide for voltammetric determination of doxorubicin. Microchim. Acta 2019, 186, 639. [Google Scholar] [CrossRef] [PubMed]
- Vacek, J.; Havran, L.; Fojta, M. Ex situ voltammetry and chronopotentiometry of doxorubicin at a pyrolytic graphite electrode: Redox and catalytic properties and analytical applications. Electroanalysis 2009, 21, 21399–22144. [Google Scholar] [CrossRef]
- Skalová, Š.; Langmaier, J.; Barek, J.; Vyskočil, V.; Navrátils, T. Doxorubicin determination using two novel voltammetric approaches: A comparative study. Electrochim. Acta 2020, 330, 135180. [Google Scholar] [CrossRef]
- Singh, T.A.; Sharma, V.; Thakur, N.; Tejwan, N.; Sharma, A.; Das, J. Selective and sensitive electrochemical detection of doxrubicin via a novel magnesium oxide/carbon dot nanocomposite based sensor. Inorg. Chem. Commun. 2023, 150, 110527. [Google Scholar] [CrossRef]
- Abbasi, M.; Ezazi, M.; Jouyban, A.; Lulek, E.; Asadpour-Zeynali, K.; Ertas, Y.N.; Houshyar, J.; Mokhtarzadeh, A.; Soleymani, J. An ultrasensitive and preprocessing-free electrochemical platform for the detection of doxorubicin based on tryptophan/polyethylene glycol-cobalt ferrite nanoparticles modified electrodes. Microchem. J. 2022, 183, 108055. [Google Scholar] [CrossRef]
- Porfireva, A.; Vorobev, V.; Babkina, S.; Evtugyn, G. Electrochemical sensor based on poly(Azure B)-DNA composite for doxorubicin determination. Sensors 2019, 19, 2085. [Google Scholar] [CrossRef]
- Peng, A.; Xu, H.; Luo, C.; Ding, H. Application of a disposable doxorubicin sensor for direct determination of clinical drug concentration in patient blood. Int. J. Electrochem. Sci. 2016, 11, 6266–6278. [Google Scholar] [CrossRef]
- Karadurmus, L.; Dogan-Topal, B.; Kurbanoglu, S.; Shah, A.; Ozkan, S.A. The interaction between DNA and three intercalating anthracyclines using electrochemical DNA nanobiosensor based on metal nanoparticles modified screen-printed electrode. Micromachines 2021, 12, 1337. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, F.H.; Taher, M.A.; Karimi-Maleh, H. Doxorubicin anticancer drug monitoring by ds-DNA-based electrochemical biosensor in clinical samples. Micromachines 2021, 12, 808. [Google Scholar] [CrossRef] [PubMed]
- Asai, K.; Yamamoto, T.; Nagashima, S.; Ogata, G.; Hibino, H.; Einaga, Y. An electrochemical aptamer-based sensor prepared by utilizing the strong interaction between a DNA aptamer and diamond. Analyst 2020, 145, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Porfireva, A.; Evtugyn, G. Electrochemical DNA sensor based on the copolymer of proflavine and Azure B for doxorubicin determination. Nanomaterials 2020, 10, 924. [Google Scholar] [CrossRef]
- Kulikova, T.; Porfireva, A.; Rogov, A.; Evtugyn, G. Electrochemical DNA sensor based on acridine yellow adsorbed on glassy carbon electrode. Sensors 2021, 21, 7763. [Google Scholar] [CrossRef]
- Shamagsumova, R.; Porfireva, A.; Stepanova, V.; Osin, Y.; Evtugyn, G.; Hianik, H. Polyaniline—DNA based sensor for the detection of anthracycline drugs. Sens. Actuators B 2015, 220, 573–582. [Google Scholar] [CrossRef]
- Evtugyn, G.A.; Porfireva, A.V.; Belyakova, S.V. Electrochemical DNA sensors for drug determination. J. Pharm. Biomed. Anal. 2022, 221, 115058. [Google Scholar] [CrossRef]







| Modifier | Concentration Range | LOD, nM | Ref. |
|---|---|---|---|
| Electrochemical sensors | |||
| Mesoporous carbon nanospheres/rGO | 10 nM–10 μM | 1.5 | [44] |
| Pyrographite | 10 nM–1.0 μM | 10 | [45] |
| Silver amalgam | 0.6–10 μM | 440 | [46] |
| /MgO/carbon nanodots/ | 0.1–1.0 μM | 90 | [47] |
| Tryptophan/PEG/CoFe2O4 | 60 nM–2.0 μM | 30 | [48] |
| Electrochemical DNA sensors | |||
| poly(Azure B) | 0.1 nM–0.1 μM | 0.07 | [49] |
| Polyaniline | 1.0 pM–1000 μM | 0.0006 | [32] |
| CNTs–polylysine | 2.5 nM–0.25 μM | 1.0 | [50] |
| Pt/Ag nanoparticles | 0.2–2.0 μM | - | [51] |
| SWCNTs | 1. nM–20 μM | 0.6 | [52] |
| BDD/DNA aptamer | Up to 2.3 μM | 49 | [53] |
| Copolymer of Azure A and proflavine | 0.03–10 nM | 0.01 | [54] |
| Acridine Yellow adsorbed on GCE | 0.1 pM–1.0 nM | 0.0007 | [55] |
| PolyPhTz/macrocyclic acids | 3.0 pM–1.0 nM | 0.001 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulikova, T.; Shiabiev, I.; Padnya, P.; Rogov, A.; Evtugyn, G.; Stoikov, I.; Porfireva, A. Impedimetric DNA Sensor Based on Electropolymerized N-Phenylaminophenothiazine and Thiacalix[4]arene Tetraacids for Doxorubicin Determination. Biosensors 2023, 13, 513. https://doi.org/10.3390/bios13050513
Kulikova T, Shiabiev I, Padnya P, Rogov A, Evtugyn G, Stoikov I, Porfireva A. Impedimetric DNA Sensor Based on Electropolymerized N-Phenylaminophenothiazine and Thiacalix[4]arene Tetraacids for Doxorubicin Determination. Biosensors. 2023; 13(5):513. https://doi.org/10.3390/bios13050513
Chicago/Turabian StyleKulikova, Tatjana, Igor Shiabiev, Pavel Padnya, Alexey Rogov, Gennady Evtugyn, Ivan Stoikov, and Anna Porfireva. 2023. "Impedimetric DNA Sensor Based on Electropolymerized N-Phenylaminophenothiazine and Thiacalix[4]arene Tetraacids for Doxorubicin Determination" Biosensors 13, no. 5: 513. https://doi.org/10.3390/bios13050513
APA StyleKulikova, T., Shiabiev, I., Padnya, P., Rogov, A., Evtugyn, G., Stoikov, I., & Porfireva, A. (2023). Impedimetric DNA Sensor Based on Electropolymerized N-Phenylaminophenothiazine and Thiacalix[4]arene Tetraacids for Doxorubicin Determination. Biosensors, 13(5), 513. https://doi.org/10.3390/bios13050513