Sensing High 17β-Estradiol Concentrations Using a Planar Microwave Sensor Integrated with a Microfluidic Channel
Abstract
:1. Introduction
2. Materials and Methods
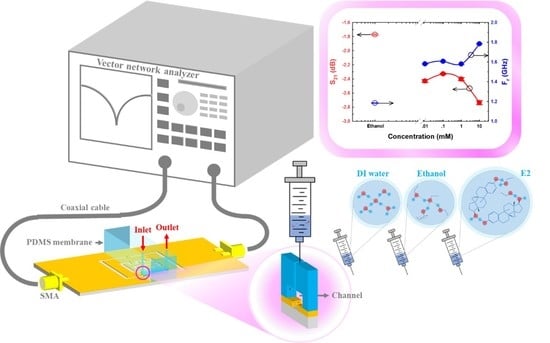
2.1. Sensor Design
2.2. Microfluidic Channel Fabrication
2.3. Preparation of Chemicals and Analyte Solutions
2.4. Sensor Fabrication and Equipment Installation
3. Results and Discussion
3.1. Sensor Response
3.2. Sensitivity Response
3.3. E2 Discrimination using Unsupervised Machine Learning
3.4. S21 and Fr Analysis
3.5. Performance Comparison of the Microwave Sensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wojnarowski, K.; Podobiński, P.; Cholewińska, P.; Smoliński, J.; Dorobisz, K. Impact of Estrogens Present in Environment on Health and Welfare of Animals. Animals 2021, 11, 2152. [Google Scholar] [CrossRef] [PubMed]
- Loutchanwoot, P.; Srivilai, P.; Jarry, H. Lack of anti-androgenic effects of equol on reproductive neuroendocrine function in the adult male rat. Horm. Behav. 2014, 65, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Loutchanwoot, P.; Vortherms, T.; Jarry, H. Evaluation of in vivo estrogenic potency of natural estrogen-active chemical, puerarin, on pituitary function in gonadectomized female rats. Life Sci. 2016, 165, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Srasri, M.; Srivilai, P.; Loutchanwoot, P. Assessment of 28-day oral exposure to Pueraria candollei var. mirifica (Fabaceae) roots on pituitary-ovarian axis function and selected metabolic parameters in ovary-intact rats. Toxicol. Rep. 2022, 9, 1831–1845. [Google Scholar] [CrossRef]
- Rechsteiner, D.; Schrade, S.; Zähner, M.; Müller, M.; Hollender, J.; Bucheli, T.D. Occurrence and Fate of Natural Estrogens in Swiss Cattle and Pig Slurry. J. Agric. Food Chem. 2020, 68, 5545–5554. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yu, W.; Yang, L.; Du, B.; Chen, S.; Sun, W.; Jiang, H.; Xie, M.; Tang, J. Occurrence and Fate of Steroid Estrogens in a Chinese Typical Concentrated Dairy Farm and Slurry Irrigated Soil. J. Agric. Food Chem. 2021, 69, 67–77. [Google Scholar] [CrossRef]
- Cummings, S.R.; Duong, T.; Kenyon, E.; Cauley, J.A.; Whitehead, M.; Krueger, K.A. Multiple Outcomes of Raloxifene Evaluation (MORE) Trial. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA 2002, 287, 216–220. [Google Scholar] [CrossRef]
- Eliassen, A.H.; Spiegelman, D.; Xu, X.; Keefer, L.K.; Veenstra, T.D.; Barbieri, R.L.; Willett, W.C.; Hankinson, S.E.; Ziegler, R.G. Urinary estrogens and estrogen metabolites and subsequent risk of breast cancer among premenopausal women. Cancer Res. 2012, 72, 696–706. [Google Scholar] [CrossRef]
- Kidd, K.A.; Blanchfield, P.J.; Mills, K.H.; Palace, V.P.; Evans, R.E.; Lazorchak, J.M.; Flick, R.W. Collapse of a fish population after exposure to a synthetic estrogen. Proc. Natl. Acad. Sci. USA 2007, 104, 8897–8901. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Z.; He, J.; Yue, X.; Pan, J.; Wang, Z. Steroidal and phenolic endocrine disrupting chemicals (EDCs) in surface water of Bahe River, China: Distribution, bioaccumulation, risk assessment and estrogenic effect on Hemiculter leucisculus. Environ. Pollut. 2018, 243 Pt A, 103–114. [Google Scholar] [CrossRef]
- Guo, T.; Gu, J.; Soldin, O.P.; Singh, R.J.; Soldin, S.J. Rapid measurement of estrogens and their metabolites in human serum by liquid chromatography-tandem mass spectrometry without derivatization. Clin. Biochem. 2008, 41, 736–741. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Arruda, A.M.M.; Pepi, G.T.; Martho, A.C.; Maximiano, P.M.; Ricci, L.S.O.B.O.; Riccio, M.F.; Noboli, A.C.; Serafim, P. Júnior. High sensitivity method validated to quantify estradiol in human plasma by LC-MS/MS. J. Chromatogr. B 2017, 1064, 109–114. [Google Scholar] [CrossRef]
- Santen, R.J.; Demers, L.; Ohorodnik, S.; Settlage, J.; Langecker, P.; Blanchett, D.; Goss, P.E.; Wang, S. Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids 2007, 72, 666–671. [Google Scholar] [CrossRef]
- Nameghi, M.A.; Danesh, N.M.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. An ultrasensitive electrochemical sensor for 17β-estradiol using split aptamers. Anal. Chim. Acta 2019, 1065, 107–112. [Google Scholar] [CrossRef]
- Jiang, Y.; Colazo, M.G.; Serpe, M.J. Poly(N-isopropylacrylamide) microgel-based etalons for the label-free quantitation of estradiol-17β in aqueous solutions and milk samples. Anal. Bioanal. Chem. 2018, 410, 4397–4407. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, W.; Ye, J.; Zhan, S.; Xia, B.; Lv, J.; Xu, H.; Du, G.; Wang, L. A label–free colorimetric biosensor for 17β-estradiol detection using nanoparticles assembled by aptamer and cationic polymer. Aust. J. Chem. 2016, 69, 12–19. [Google Scholar] [CrossRef]
- Huang, H.; Shi, S.; Gao, X.; Gao, R.; Zhu, Y.; Wu, X.; Zang, R.; Yao, T. A universal label-free fluorescent aptasensor based on Ru complex and quantum dots for adenosine, dopamine and 17β-estradiol detection. Biosens. Bioelectron. 2016, 79, 198–204. [Google Scholar] [CrossRef]
- Ni, X.; Xia, B.; Wang, L.; Ye, J.; Du, G.; Feng, H.; Zhou, X.; Zhang, T.; Wang, W. Fluorescent aptasensor for 17β-estradiol determination based on gold nanoparticles quenching the fluorescence of Rhodamine B. Anal. Biochem. 2017, 523, 17–23. [Google Scholar] [CrossRef]
- Liu, J.; Bai, W.; Niu, S.; Zhu, C.; Yang, S.; Chen, A. Highly sensitive colorimetric detection of 17 β-estradiol using split DNA aptamers immobilized on unmodified gold nanoparticles. Sci. Rep. 2014, 4, 7571–7576. [Google Scholar] [CrossRef]
- Liu, P.P.; Liu, X.; Huo, X.H.; Tang, Y.; Xu, J.; Ju, H. TiO2–BiVO4 heterostructure to enhance photoelectrochemical efficiency for sensitive aptasensing. ACS Appl. Mater. Interfaces 2017, 9, 27185–27192. [Google Scholar] [CrossRef]
- Waifalkar, P.P.; Noh, D.; Derashri, P.; Barage, S.; Oh, E. Role of estradiol hormone in human life and electrochemical aptasensing of 17β-estradiol: A review. Biosensors 2022, 12, 1117. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Zhi, H.; Keefe, D.W.; Gao, B.; LeFevre, G.H.; Toor, F. Sensitive and specific detection of estrogens featuring doped silicon nanowire arrays. ACS Omega 2022, 7, 47341–47348. [Google Scholar] [CrossRef] [PubMed]
- Pu, H.; Huang, Z.; Sun, D.W.; Fu, H. Recent advances in the detection of 17β-estradiol in food matrices: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2144–2157. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Zhao, X.; Guo, J.; Feng, S.; Fu, Y.; Kang, Y.; Guo, J. Microfluidics-based microwave sensor. Sens. Actuators A 2020, 309, 111910. [Google Scholar] [CrossRef]
- Alahnomi, R.A.; Zakaria, Z.; Yussof, Z.M.; Althuwayb, A.A.; Alhegazi, A.; Alsariera, H.; Rahman, N.A. Review of recent microwave planar resonator-based sensors: Techniques of complex permittivity extraction, applications, open challenges and future research directions. Sensors 2021, 21, 2267. [Google Scholar] [CrossRef]
- Mohammadi, S.; Adhikari, K.K.; Jain, M.C.; Zarifi, M.H. High-resolution, sensitivity-enhanced active resonator sensor using substrate-embedded channel for characterizing low-concentration liquid mixtures. IEEE Trans. Microw. Theory Tech. 2022, 70, 576–586. [Google Scholar] [CrossRef]
- Camli, B.; Altinagac, E.; Kizil, H.; Torun, H.; Dundar, G.; Yalcinkaya, A.D. Gold-on-glass microwave split-ring resonators with PDMS microchannels for differential measurement in microfluidic sensing. Biomicrofluidics 2020, 14, 054102. [Google Scholar] [CrossRef]
- Mohammadi, S.; Wiltshire, B.; Jain, M.C.; Nadaraja, A.V.; Clements, A.; Golovin, K.; Roberts, D.J.; Johnson, T.; Foulds, I.; Zarifi, M.H. Gold coplanar waveguide resonator integrated with a microfluidic channel for aqueous dielectric detection. IEEE Sens. J. 2020, 20, 9825–9833. [Google Scholar] [CrossRef]
- Mohammadi, S.; Nadaraja, A.V.; Roberts, D.J.; Zarifi, M.H. Real-time and hazard-free water quality monitoring based on microwave planar resonator sensor. Sens. Actuators A 2020, 303, 111663. [Google Scholar] [CrossRef]
- Chretiennot, T.; Dubuc, D.; Grenier, K. A microwave and microfluidic planar resonator for efficient and accurate complex permittivity characterization of aqueous solutions. IEEE Trans. Microw. Theory Tech. 2013, 61, 972–978. [Google Scholar] [CrossRef]
- Abduljabar, A.A.; Rowe, D.J.; Porch, A.; Barrow, D.A. Novel microwave microfluidic sensor using a microstrip split-ring resonator. IEEE Trans. Microw. Theory Tech. 2014, 62, 679–688. [Google Scholar] [CrossRef]
- Awang, R.A.; Tovar-Lopez, F.J.; Baum, T.; Sriram, S.; Rowe, W.S.T. Meta-atom microfluidic sensor for measurement of dielectric properties of liquids. J. Appl. Phys. 2017, 121, 094506–8. [Google Scholar] [CrossRef]
- Bahar, A.A.M.; Zakaria, Z.; Arshad, M.K.M.; Lsa, A.A.M.; Dasril, Y.; Alahnomi, R.A. Real time microwave biochemical sensor based on circular SIW approach for aqueous dielectric detection. Sci. Rep. 2019, 9, 5467. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Scott, J.; Ghorbani, K. Ultrahigh-sensitivity microwave sensor for microfluidic complex permittivity measurement. IEEE Trans. Microw. Theory Tech. 2019, 67, 4269–4277. [Google Scholar] [CrossRef]
- Vélez, P.; Su, L.; Grenier, K.; Mata-Contreras, J.; Dubuc, D.; Martín, F. Microwave microfluidic sensor based on a microstrip splitter/combiner configuration and split ring resonators (SRRs) for dielectric characterization of liquids. IEEE Sens. J. 2017, 17, 6589–6598. [Google Scholar] [CrossRef]
- Wiltshire, B.D.; Zarifi, M.H. 3-D printing microfluidic channels with embedded planar microwave resonators for RFID and liquid detection. IEEE Microw. Wirel. Compon. Lett. 2019, 29, 65–67. [Google Scholar] [CrossRef]
- Salim, A.; Lim, S. Complementary split-ring resonator-loaded microfluidic ethanol chemical sensor. Sensors 2016, 16, 1802. [Google Scholar] [CrossRef]
- Withayachumnankul, W.; Jaruwongrungsee, K.; Tuantranont, A.; Fumeaux, C.; Abbott, D. Metamaterial-based microfluidic sensor for dielectric characterization. Sens. Actuators A 2013, 189, 233–237. [Google Scholar] [CrossRef]
- Chuma, E.L.; Iano, Y.; Fontgalland, G.; Roger, L.L.B. Microwave sensor for liquid dielectric characterization based on metamaterial complementary split ring resonator. IEEE Sens. J. 2018, 18, 9978–9983. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Withayachumnankul, W.; Al-Sarawi, S.; Abbott, D. High-sensitivity metamaterial-inspired sensor for microfluidic dielectric characterization. IEEE Sens. J. 2014, 14, 1345–1351. [Google Scholar] [CrossRef]
- Zhao, W.S.; Wang, B.X.; Wang, D.W.; You, B.; Liu, Q.; Wang, G. Swarm intelligence algorithm-based optimal design of microwave microfluidic sensors. IEEE Trans. Ind. Electron. 2022, 69, 2077–2087. [Google Scholar] [CrossRef]
- Gan, H.Y.; Zhao, W.S.; Liu, Q.; Wang, D.W.; Dong, L.; Wang, G.; Yin, W.Y. Differential microwave microfluidic sensor based on microstrip complementary split-ring resonator (MCSRR) structure. IEEE Sens. J. 2020, 20, 5876–5884. [Google Scholar] [CrossRef]
- Fan, L.-C.; Zhao, W.-S.; Wang, D.-W.; Liu, Q.; Chen, S.; Wang, G. An ultrahigh sensitivity microwave sensor for microfluidic applications. IEEE Microw. Wirel. Compon. Lett. 2020, 30, 1201–1204. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Scott, J.; Ghorbani, K. Microwave reflective biosensor for glucose level detection in aqueous solutions. Sens. Actuators A 2020, 301, 111662. [Google Scholar] [CrossRef]
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Alquie, G.; Deshours, F.; Kokabi, H.; Shubair, R.M. Non-invasive real-time monitoring of glucose level using novel microwave biosensor based on triple-pole CSRR. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 1407–1420. [Google Scholar] [CrossRef]
- Jang, C.; Park, J.K.; Lee, H.J.; Yun, G.H.; Yook, J.G. Sensitivity-enhanced fluidic glucose sensor based on a microwave resonator coupled with an interferometric system for noninvasive and continuous detection. IEEE Trans. Biomed. Circuits Syst. 2021, 15, 1017–1026. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.H.; Moon, H.S.; Jang, I.S.; Choi, J.S.; Yook, J.G.; Jung, H.I. A planar split-ring resonator-based microwave biosensor for label-free detection of biomolecules. Sens. Actuators B 2012, 169, 26–31. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, H.S.; Yoo, K.H.; Yook, J.G. DNA sensing using split-ring resonator alone at microwave regime. J. Appl. Phys. 2010, 108, 014908–6. [Google Scholar] [CrossRef]
- Lee, H.J.; Yook, J.G. Biosensing using split-ring resonators at microwave regime. Appl. Phys. Lett. 2008, 92, 254103–3. [Google Scholar] [CrossRef]
- Srisai, S.; Harnsoongnoen, S. Noncontact planar microwave sensor for liquid interface detection by a pixelated CSRR-loaded microstrip line. Int. J. RF Microw. Comput. Aided Eng. 2021, 31, e22557. [Google Scholar] [CrossRef]
- Loutchanwoot, P.; Harnsoongnoen, S. Microwave microfluidic sensor for detection of high equol concentrations in aqueous solution. IEEE Trans. Biomed. Circuits Syst. 2022, 16, 244–251. [Google Scholar] [CrossRef]
- Saadat-Safa, M.; Nayyeri, V.; Ghadimi, A.; Soleimani, M.; Ramahi, O.M. A pixelated microwave near-field sensor for precise characterization of dielectric materials. Sci. Rep. 2019, 9, 13310. [Google Scholar] [CrossRef]
- Ali, J.K.; Alsaedi, H.; Hasan, M.F.; Hammas, H.A. A Peano fractal-based dual-mode microstrip bandpass filters for wireless communication systems. In Proceedings of the Progress in Electromagnetics Research Symposium, PIERS, Moscow, Russia, 19–23 August 2012. [Google Scholar]
- Harnsoongnoen, S. Metamaterial-inspired microwave sensor for detecting the concentration of mixed phosphate and nitrate in water. IEEE Trans. Instrum. Meas. 2021, 70, 9509906. [Google Scholar] [CrossRef]
- Bonache, J.; Gil, M.; Gil, I.; García-García, J.; Martín, F. On the electrical characteristics of complementary metamaterial resonators. IEEE Microw. Wirel. Compon. Lett. 2006, 16, 543–545. [Google Scholar] [CrossRef]
- Castro-Giráldez, M.; Fito, P.J.; Fito, P. Application of microwaves dielectric spectroscopy for controlling pork meat (Longissimus dorsi) salting process. J. Food Eng. 2010, 97, 484–490. [Google Scholar] [CrossRef]
- Lauzon, S.D.; Rajkowski, K.M.; Cittanova, N. Investigation of a 17β-Estratiol-monoclonal antiestradiol antibody binding mechanism using dilute solutions of organic solvents. J. Steroid Biochem. Mol. Biol. 1994, 48, 225–233. [Google Scholar] [CrossRef]
- Chan, K.Y.; Courtois, B.; Loose, K.; Hare, P.M. Solvent-dependent fluorescence lifetimes of estrone, 17β-Estradiol and 17β-Ethinylestradiol. Photochem. Photobiol. 2013, 89, 294–299. [Google Scholar] [CrossRef]
- Stevenson, E.L.; Lancaster, R.W.; Buanz, A.B.M.; Price, L.S.; Tocher, D.A.; Price, S.L. The solid-state forms of the sex hormone 17-β-estradiol. Cryst. Eng. Comm. 2019, 21, 2154–2163. [Google Scholar] [CrossRef]
- DeMello, A.J. I’m Sensitive about Sensitivity. ACS Sens. 2022, 7, 1235–1236. [Google Scholar] [CrossRef]
- Indrayanto, G. Validation of chromatographic methods of analysis: Application for drugs that derived from herbs. Profiles Drug Subst. Excip. Relat. Methodol. 2018, 43, 359–392. [Google Scholar]
- Dey, G.; Venkateswarulu, M.; Vivekananthan, V.; Pramanik, A.; Krishnan, V.; Koner, R.R. Sub-picomolar recognition of Cr3+ through bioinspired organic–inorganic ensemble utilization. ACS Sens. 2016, 1, 663–669. [Google Scholar] [CrossRef]
- Purusothaman, Y.; Alluri, N.R.; Chandrasekhar, A.; Venkateswaran, A.; Kim, S.J. Piezophototronic gated optofluidic logic computations empowering intrinsic reconfigurable switches. Nat. Commun. 2019, 10, 4381. [Google Scholar] [CrossRef] [PubMed]
- Vivekananthan, V.; Alluri, N.R.; Purusothaman, Y.; Chandrasekhar, A.; Selvarajan, S.; Kim, S.J. Biocompatible collagen nanofibrils: An approach for sustainable energy harvesting and battery-free humidity sensor applications. ACS Appl. Mater. Interfaces 2018, 10, 18650–18656. [Google Scholar] [CrossRef] [PubMed]
- Mohsen-Nia, M.; Amiri, H.; Jazi, B. Dielectric constants of water, methanol, ethanol, butanol and acetone: Measurement and computational study. J. Solut. Chem. 2010, 39, 701–708. [Google Scholar] [CrossRef]
- Oren, I.; Fleishman, S.J.; Kessel, A.; Ben-Tal, N. Free diffusion of steroid hormones across biomembranes: A simplex search with implicit solvent model calculations. Biophys. J. 2004, 87, 768–779. [Google Scholar] [CrossRef]
- Zhuang, B.; Ramanauskaite, G.; Koa, Z.Y.; Wang, Z.G. Like dissolves like: A first-principles theory for predicting liquid miscibility and mixture dielectric constant. Sci. Adv. 2021, 7, eabe7275. [Google Scholar] [CrossRef]
- Schäfer, A.I.; Akanyeti, I.; Semião, A.J.C. Micropollutant sorption to membrane polymers: A review of mechanisms for estrogens. Adv. Colloid Interface Sci. 2011, 164, 100–117. [Google Scholar] [CrossRef]
- Arik, E.; Altan, H.; Esenturk, O. Dielectric properties of ethanol and gasoline mixtures by terahertz spectroscopy and an effective method for determination of ethanol content of gasoline. J. Phys. Chem. A 2014, 118, 3081–3089. [Google Scholar] [CrossRef]














| Parameter | W | L | c | s | l | g |
|---|---|---|---|---|---|---|
| Value (mm) | 1.3 | 11 | 0.5 | 0.2 | 4.5 | 0.2 |
| Dependent Variables | Regression Equations | R2 |
|---|---|---|
| S21 | S21 = −0.0362C − 2.3746 | 0.9433 |
| Fr | Fr = 1.96 × 106C + 1.58 × 109 | 0.9646 |
| Ref. | Sensors | Spec. | Conc. | Data | Volume (µL) | LOD | Linear Range | Time Required |
|---|---|---|---|---|---|---|---|---|
| [14] | Electro-chemical aptasensor | E2, milk, serum | 0–15 nM | ΔI | 10 | 0.5 pM | 1.5–100 pM and 100–7000 pM | ~30 min |
| [15] | Aptasensor based on pNIPAm microgel-based etalons | E2, milk | 0–734 pM | Δλ | NA | 3.2 pM | 3.2–640 pM | ~30 min |
| [16] | Colorimetric aptasensor based on gold nanoparticle with DNA aptamer | E2 | 0–4.5 µM | ΔA | 500 | 1.57 nM | 1.57–350 nM | ~30 min |
| [17] | Fluorescent aptasensor based on Ru complex and quantum dots | ADE, DA, E2 | 0–400 nM | ΔF | NA | 37 nM | 0.08–0.4 µM | ~1 h |
| [18] | Fluorescent aptasensor based on gold nanoparticle and RhoB | E2 | 0–1.5 µM | ΔF | >100 | 0.48 nM | 0.48–200 nM | ~30 min |
| [19] | Colorimetric aptasensor based on DNA aptamer and gold nanoparticle | E2 | 0–370 µM | ΔA | 160 | ~367 pM | ~0.367–367,000 nM | ~35 min |
| [20] | PEC and DNA aptamer and TiO2-BiVO4 | E2 | 0–250 pM | ΔI | NA | 22 fM | 0.1–250 pM | >1 h |
| TW | PF-NSCSRR | Free space, DI water, Ethanol, E2 | 0–10 mM | Fr, S21 | 1.37 | 3.4 mM | 0.001–10 mM | <1 min |
| Ref. | Structure | Spec. | Conc. | Meth. | Fr (GHz) | Sensitivity | Volume (μL) | Channel Area (mm2) |
|---|---|---|---|---|---|---|---|---|
| [30] | CPW resonator | Ethanol | 0–20% by volume | Fr, S21 | 20 | −63.1 MHz; 3.56 × 10−3 | 2.95 × 10−3 | 0.074 |
| [34] | Series LC | Ethanol | 0–100% by volume | Fr, S21 | 1.91 | 0.45% | 0.637 | 9.1 |
| [37] | CSRR | Ethanol | 0–100% by volume | Fr | 4.72 | 49.1 MHz | 3 | 5 |
| [38] | SRR | Ethanol, Methanol | 0–100% by volume | Fr | 2.1 | NA | 0.108 | 1.8 |
| [39] | CSRR | Ethanol | 0–100% by volume | Fr | 2.3 | NA | 236 | 0.79 |
| [40] | CSRR | Ethanol | 0–100% by volume | Fr | 2.02 | 0.308% | 0.588 | 0.042 |
| [41] | Series LC | Ethanol | 0–100% by volume | Fr | 1.662 | 0.695% | 0.7 | 0.02 |
| [42] | MCSRR | Ethanol | 0–100% by volume | Fr | 1.62 | 0.469% | 5.4 | 0.2 |
| [43] | CSRR | Ethanol | 0–100% by volume | Fr | 2.226 | 0.62% | 0.52 | 0.02 |
| [44] | CSRR | Glucose | 0–80 mg/mL | Fr | 2.48 | 0.5 × 10−3 MHz and 0.5 dB | 0.637 | 9.1 |
| [45] | TP-CSRR | Glucose | 0.7–1.2 mg/mL | S21, S11 | 2.3 | 1.7–6.2 dB (S21) and 0.6–3.45 dB (S11) | 648 | 324 |
| [46] | CSRR-interferometric system | Glucose | 0–400 mg/dL | Fr, S21 | 2.26 | 1.947 mdB | 6.9 | 11.06 |
| TW | PF-NSCSRR | Ethanol, E2 | 0–10 mM | Fr, S21 | 2.30 | 40 GHz (Fr) and 1746.98 dB (S21) | 1.37 | 2.7 |
| Ref. | Structure | Spec. | Conc. | Meth. | Fr (GHz) | Sensitivity | Q-Factor | Channel Area (mm2) | Volume (μL) |
|---|---|---|---|---|---|---|---|---|---|
| [51] | PF-CSRR | DMSO, EQ | 0–100 mM | Fr, S21 | 2.34 | 61.97 GHz (Fr) and 1646.87 dB (S21) | 81.64 | 3.6 | 1.91 |
| [52] | Pixelated pattern resonator | Cyclohexane (C6H12), Chloroform (CHCl3) | 0–100% | Fr, S21 | 5.64 | NA | NA | 104.04 | NA |
| [54] | SCSRR | Phosphate, Nitrate | 0–1 mg/mL | Fr, S21 | 2.33 | 241 MHz (Fr) and 3.21 dB (S21) | 95.00 | 12.57 | 100 |
| TW | PF-NSCSRR | Ethanol, E2 | 0–10 mM | Fr, S21 | 2.30 | 40 GHz (Fr) and 1746.98 dB (S21) | 114.88 | 2.7 | 1.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harnsoongnoen, S.; Loutchanwoot, P.; Srivilai, P. Sensing High 17β-Estradiol Concentrations Using a Planar Microwave Sensor Integrated with a Microfluidic Channel. Biosensors 2023, 13, 541. https://doi.org/10.3390/bios13050541
Harnsoongnoen S, Loutchanwoot P, Srivilai P. Sensing High 17β-Estradiol Concentrations Using a Planar Microwave Sensor Integrated with a Microfluidic Channel. Biosensors. 2023; 13(5):541. https://doi.org/10.3390/bios13050541
Chicago/Turabian StyleHarnsoongnoen, Supakorn, Panida Loutchanwoot, and Prayook Srivilai. 2023. "Sensing High 17β-Estradiol Concentrations Using a Planar Microwave Sensor Integrated with a Microfluidic Channel" Biosensors 13, no. 5: 541. https://doi.org/10.3390/bios13050541
APA StyleHarnsoongnoen, S., Loutchanwoot, P., & Srivilai, P. (2023). Sensing High 17β-Estradiol Concentrations Using a Planar Microwave Sensor Integrated with a Microfluidic Channel. Biosensors, 13(5), 541. https://doi.org/10.3390/bios13050541