Fe3O4@Au Core–Shell Magnetic Nanoparticles for the Rapid Analysis of E. coli O157:H7 in an Electrochemical Immunoassay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Equipment, Reagents, and Solutions
2.2. Preparation of E. coli O157:H7
2.3. Fe3O4@Au Synthesis and Functionalization
2.4. Magnetic Nanoparticles Characterization
2.5. Immobilization of Capture Antibody on Fe3O4@Au
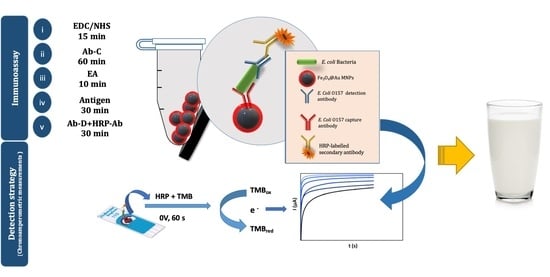
2.6. Immunoassay Procedure
2.7. Milk Sample Analysis
3. Results and Discussion
3.1. Fe3O4@Au MNP Synthesis and Characterization
3.2. Optimization of Experimental Conditions
3.3. Analytical Performance of the Immunoassay for E. coli O157:H7 Analysis
3.4. Milk Sample Analysis
3.5. Comparison with Other Electrochemical Immunoassays for the Determination of E. coli
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, B.; Liu, Y.; Yang, B.; Wang, Q.; Liu, X.; Qin, J.; Zhao, K.; Li, F.; Feng, X.; Li, L.; et al. Escherichia coli O157:H7 Senses Microbiota-Produced Riboflavin to Increase Its Virulence in the Gut. Proc. Natl. Acad. Sci. USA 2022, 119, e2212436119. [Google Scholar] [CrossRef]
- Gambushe, S.M.; Zishiri, O.T.; El Zowalaty, M.E. Review of Escherichia coli O157:H7 Prevalence, Pathogenicity, Heavy Metal and Antimicrobial Resistance, African Perspective. Infect. Drug Resist. 2022, 15, 4645–4673. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO [Food and Agriculture Organization of the Organization]. Enterohaemorrhagic Escherichia coli in Raw Beef and Beef Products: Approaches for the Provision of Scientific Advice: Meeting Report; Microbiological Risk Assessment Series 18; World Health Organization: Geneva, Switzerland, 2011; p. 126. [Google Scholar]
- Griffin, P.M.; Ostroff, S.M.; Tauxe, R.V.; Greene, K.D.; Wells, J.G.; Lewis, J.H.; Blake, P.A. Illnesses Associated with Escherichia coli 0157:H7 Infections: A Broad Clinical Spectrum. Ann. Intern. Med. 1988, 109, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Pebdeni, A.B.; Roshani, A.; Mirsadoughi, E.; Behzadifar, S.; Hosseini, M. Recent Advances in Optical Biosensors for Specific Detection of E. coli Bacteria in Food and Water. Food Control 2022, 135, 108822. [Google Scholar] [CrossRef]
- Jang, J.; Hur, H.G.; Sadowsky, M.J.; Byappanahalli, M.N.; Yan, T.; Ishii, S. Environmental Escherichia coli: Ecology and Public Health Implications—A Review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wu, Y.; Li, Y.; Hong, Y.; Zhao, X.; Reyes, P.I.; Lu, Y. Early Stage Detection of Staphylococcus Epidermidis Biofilm Formation Using MgZnO Dual-Gate TFT Biosensor. Biosens. Bioelectron. 2020, 151, 111993. [Google Scholar] [CrossRef]
- Quintela, I.A.; Vasse, T.; Lin, C.-S.; Wu, V.C.H. Advances, Applications, and Limitations of Portable and Rapid Detection Technologies for Routinely Encountered Foodborne Pathogens. Front. Microbiol. 2022, 13, 1054782. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Janik-Karpinska, E.; Ceremuga, M.; Niemcewicz, M.; Podogrocki, M.; Stela, M.; Cichon, N.; Bijak, M. Immunosensors—The Future of Pathogen Real-Time Detection. Sensors 2022, 22, 9757. [Google Scholar] [CrossRef]
- Fei, J.; Dou, W.; Zhao, G. Amperometric Immunoassay for the Detection of Salmonella Pullorum Using a Screen—Printed Carbon Electrode Modified with Gold Nanoparticle-Coated Reduced Graphene Oxide and Immunomagnetic Beads. Microchim. Acta 2016, 183, 757–764. [Google Scholar] [CrossRef]
- Pei, X.; Zhang, B.; Tang, J.; Liu, B.; Lai, W.; Tang, D. Sandwich-Type Immunosensors and Immunoassays Exploiting Nanostructure Labels: A Review. Anal. Chim. Acta 2013, 758, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Loo, K.Y.; Law, J.W.F.; Tan, L.T.H.; Pusparajah, P.; Letchumanan, V.; Lee, L.H. Diagnostic Techniques for Rapid Detection of Vibrio Species. Aquaculture 2022, 561, 738628. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Magnetic Particles Coupled to Disposable Screen Printed Transducers for Electrochemical Biosensing. Sensors 2016, 16, 1585. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Li, C.; Yang, X.; Wang, S.; Zhang, X.; Zhao, C.; Xue, B.; Gao, C.; Zhou, H.; Yang, Y.; et al. Rapid Quantitative Detection of Live Escherichia coli Based on Chronoamperometry. Biosensors 2022, 12, 845. [Google Scholar] [CrossRef] [PubMed]
- Phasuksom, K.; Sirivat, A. Chronoampermetric Detection of Enzymatic Glucose Sensor Based on Doped Polyindole/MWCNT Composites Modified onto Screen-Printed Carbon Electrode as Portable Sensing Device for Diabetes. RSC Adv. 2022, 12, 28505–28518. [Google Scholar] [CrossRef]
- Hassan, R.Y.A. Advances in Electrochemical Nano-Biosensors for Biomedical and Environmental Applications: From Current Work to Future Perspectives. Sensors 2022, 22, 7539. [Google Scholar] [CrossRef]
- Pan, M.; Gu, Y.; Yun, Y.; Li, M.; Jin, X.; Wang, S. Nanomaterials for Electrochemical Immunosensing. Sensors 2017, 17, 1041. [Google Scholar] [CrossRef]
- Freitas, M.; Sá Couto, M.; Barroso, M.F.; Pereira, C.; De-Los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J.; Delerue-Matos, C. Highly Monodisperse Fe3O4@Au Superparamagnetic Nanoparticles as Reproducible Platform for Genosensing Genetically Modified Organisms. ACS Sens. 2016, 1, 1044–1053. [Google Scholar] [CrossRef]
- Bazsefidpar, S.; Moyano, A.; Gutiérrez, G.; Matos, M.; Blanco-López, M.C. Lipid–Polymer Hybrids Encapsulating Iron-Oxide Nanoparticles as a Label for Lateral Flow Immunoassays. Biosensors 2021, 11, 218. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, C.; Zhang, Z.; Wu, W.; Wang, X.; Yu, Z. Synthesis, Functionalization, and Nanomedical Applications of Functional Magnetic Nanoparticles. Chin. Chem. Lett. 2018, 29, 1601–1608. [Google Scholar] [CrossRef]
- Moraes Silva, S.; Tavallaie, R.; Sandiford, L.; Tilley, R.D.; Gooding, J.J. Gold Coated Magnetic Nanoparticles: From Preparation to Surface Modification for Analytical and Biomedical Applications. Chem. Commun. 2016, 52, 7528–7540. [Google Scholar] [CrossRef] [PubMed]
- Fanjul-Bolado, P.; González-García, M.B.; Costa-García, A. Amperometric Detection in TMB/HRP-Based Assays. Anal. Bioanal. Chem. 2005, 382, 297–302. [Google Scholar] [CrossRef]
- Xu, Z.; Shen, C.; Hou, Y.; Gao, H.; Sun, S. Oleylamine as Both Reducing Agent and Stabilizer in a Facile Synthesis of Magnetite Nanoparticles. Chem. Mater. 2009, 21, 1778–1780. [Google Scholar] [CrossRef]
- Freitas, M.; Nouws, H.P.A.; Keating, E.; Delerue-Matos, C. High-Performance Electrochemical Immunomagnetic Assay for Breast Cancer Analysis. Sens. Actuators B Chem. 2020, 308, 127667. [Google Scholar] [CrossRef]
- Freitas, M.; Nouws, H.P.A.; Keating, E.; Fernandes, V.C.; Delerue-Matos, C. Immunomagnetic Bead-Based Bioassay for the Voltammetric Analysis of the Breast Cancer Biomarker HER2-ECD and Tumour Cells Using Quantum Dots as Detection Labels. Microchim. Acta 2020, 187, 184. [Google Scholar] [CrossRef]
- Wang, R.; Lum, J.; Callaway, Z.; Lin, J.; Bottje, W.; Li, Y. A Label-Free Impedance Immunosensor Using Screen-Printed Interdigitated Electrodes and Magnetic Nanobeads for the Detection of E. coli O157:H7. Biosensors 2015, 5, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Jantra, J.; Kanatharana, P.; Asawatreratanakul, P.; Hedstrom, M.; Mattiasson, B.; Thavarungkul, P. Real-Time Label-Free Affinity Biosensors for Enumeration of Total Bacteria Based on Immobilized Concanavalin A. J. Environ. Sci. Health A 2011, 46, 1450–1460. [Google Scholar] [CrossRef]
- Wang, Y.; Ping, J.; Ye, Z.; Wu, J.; Ying, Y. Impedimetric Immunosensor Based on Gold Nanoparticles Modified Graphene Paper for Label-Free Detection of Escherichia coli O157: H7. Biosens. Bioelectron. 2013, 49, 492–498. [Google Scholar] [CrossRef]
- Li, Z.; Fu, Y.; Fang, W.; Li, Y. Electrochemical Impedance Immunosensor Based on Self-Assembled Monolayers for Rapid Detection of Escherichia coli O157:H7 with Signal Amplification Using Lectin. Sensors 2015, 15, 19212–19224. [Google Scholar] [CrossRef]
- Wan, J.; Ai, J.; Zhang, Y.; Geng, X.; Gao, Q.; Cheng, Z. Signal-off Impedimetric Immunosensor for the Detection of Escherichia coli O157:H7. Sci. Rep. 2016, 6, 19806. [Google Scholar] [CrossRef]
- Chan, K.Y.; Ye, W.W.; Zhang, Y.; Xiao, L.D.; Leung, P.H.M.; Li, Y.; Yang, M. Ultrasensitive Detection of E. coli O157:H7 with Biofunctional Magnetic Bead Concentration via Nanoporous Membrane Based Electrochemical Immunosensor. Biosens. Bioelectron. 2013, 41, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Pandey, C.M.; Pandey, M.K.; Sumana, G. Langmuir–Blodgett Based Ordered Deposition of Functionalized Iron Oxide Nanoparticles for Ultrasensitive Detection of Escherichia coli O157: H7. Microchem. J. 2022, 181, 107708. [Google Scholar] [CrossRef]





| Average Particle Size | ||||
|---|---|---|---|---|
| MNP | DTEM (nm) | σ(TEM) c | DDLS (nm) d | PdI e |
| Fe3O4 | 5.4 a | 1.0 | 5.6 | 0.145 |
| Fe3O4@Au | 13.7 b | 1.9 nm | 15.7 | 0.076 |
| [E. coli] Added (CFU/mL) | [E. coli] Found (CFU/mL) | Recovery (%) a | CV (%) b |
|---|---|---|---|
| 0 | <LOD | ||
| 2 × 101 | <LOD | ||
| 2 × 102 | 2.5 × 102 | 125 | 2.2 |
| 2 × 103 | 2.3 × 103 | 115 | 1.7 |
| Target Bacteria | Transducer | Modification/Platform | Label/Redox Probe | Technique | Assay Time | LOD (CFU/mL) | Ref. |
|---|---|---|---|---|---|---|---|
| E. coli | Glassy carbon electrode | - | [Fe(CN)6]4− | Chronoamperometry | <5 min | 1 × 104 | [15] |
| E. coli O157:H7 | SPCE | Magnetic nanobeads | Label-free | EIS a | <1 h | 1 × 104 | [27] |
| E. coli | Gold disk electrode | - | Label-free | EIS a | <20 min | 6 × 103 | [28] |
| E. coli O157:H7 | Graphene paper electrode | AuNPs | Label-free | EIS a | - | 1.5 × 102 | [29] |
| E. coli O157:H7 | Screen-printed interdigitated microelectrodes | - | [Fe(CN)6]3−/4− | EIS a | <1 h | 1 × 102 | [30] |
| E. coli O157:H7 | SAM-modified gold electrodes | AuNPs | [Fe(CN)6]3−/4− | EIS a | - | 1 × 102 | [31] |
| E. coli O157:H7 | Nanoporous membrane | Magnetic beads | Label-free | EIS a | - | 10 | [32] |
| E. coli O157:H7 | ITO c | Plg-IONPs b | [Fe(CN)6]3−/4− | EIS a | - | 3 | [33] |
| E. coli O157:H7 | SPCE | Fe3O4@Au MNPs | HRP | Chronoamperometry | 1 h | 20 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazsefidpar, S.; Freitas, M.; Pereira, C.R.; Gutiérrez, G.; Serrano-Pertierra, E.; Nouws, H.P.A.; Matos, M.; Delerue-Matos, C.; Blanco-López, M.C. Fe3O4@Au Core–Shell Magnetic Nanoparticles for the Rapid Analysis of E. coli O157:H7 in an Electrochemical Immunoassay. Biosensors 2023, 13, 567. https://doi.org/10.3390/bios13050567
Bazsefidpar S, Freitas M, Pereira CR, Gutiérrez G, Serrano-Pertierra E, Nouws HPA, Matos M, Delerue-Matos C, Blanco-López MC. Fe3O4@Au Core–Shell Magnetic Nanoparticles for the Rapid Analysis of E. coli O157:H7 in an Electrochemical Immunoassay. Biosensors. 2023; 13(5):567. https://doi.org/10.3390/bios13050567
Chicago/Turabian StyleBazsefidpar, Shayesteh, Maria Freitas, Clara R. Pereira, Gemma Gutiérrez, Esther Serrano-Pertierra, Henri P. A. Nouws, María Matos, Cristina Delerue-Matos, and María Carmen Blanco-López. 2023. "Fe3O4@Au Core–Shell Magnetic Nanoparticles for the Rapid Analysis of E. coli O157:H7 in an Electrochemical Immunoassay" Biosensors 13, no. 5: 567. https://doi.org/10.3390/bios13050567
APA StyleBazsefidpar, S., Freitas, M., Pereira, C. R., Gutiérrez, G., Serrano-Pertierra, E., Nouws, H. P. A., Matos, M., Delerue-Matos, C., & Blanco-López, M. C. (2023). Fe3O4@Au Core–Shell Magnetic Nanoparticles for the Rapid Analysis of E. coli O157:H7 in an Electrochemical Immunoassay. Biosensors, 13(5), 567. https://doi.org/10.3390/bios13050567