Probing Contaminant-Induced Alterations in Chlorophyll Fluorescence by AC-Dielectrophoresis-Based 2D-Algal Array
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Cell Cultures and Test Media
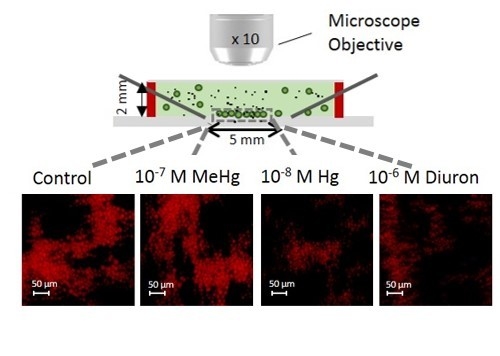
2.2. Measurements of Chlorophyll Fluorescence by 2D Arrays Combined with Fluorescence Microscopy
2.3. Measurements of Chlorophyll Fluorescence by Flow Cytometry
2.4. Chlorophyll Fluorescence Changes upon Contaminant Exposure
2.5. Statistical Treatments
3. Results and Discussion
3.1. Effect of DEP on Chlorophyll Fluorescence
3.2. Effect of CuO-NPs and Cu on Chlorophyll Fluorescence
3.3. 2D-Assembly Based Sensing of Fluorescence during Short-Term Exposure to Contaminants
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Rohacek, K.; Bartak, M. Technique of the modulated chlorophyll fluorescence: Basic concepts, useful parameters, and some applications. Photosynthetica 1999, 37, 339–363. [Google Scholar] [CrossRef]
- Kumar, K.S.; Dahms, H.U.; Lee, J.S.; Kim, H.C.; Lee, W.C.; Shin, K.H. Algal photosynthetic responses to toxic metals and herbicides assessed by chlorophyll a fluorescence. Ecotoxicol. Environ. Saf. 2014, 104, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Buonasera, K.; Lambreva, M.; Rea, G.; Touloupakis, E.; Giardi, M.T. Technological applications of chlorophyll a fluorescence for the assessment of environmental pollutants. Anal. Bioanal. Chem. 2011, 401, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Brayner, R.; Coute, A.; Livage, J.; Perrette, C.; Sicard, C. Micro-algal biosensors. Anal. Bioanal. Chem. 2011, 401, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Frense, D.; Muller, A.; Beckmann, D. Detection of environmental pollutants using optical biosensor with immobilized algae cells. Sens. Actuators B Chem. 1998, 51, 256–260. [Google Scholar] [CrossRef]
- Franqueira, D.; Orosa, M.; Torres, E.; Herrero, C.; Cid, A. Potential use of flow cytometry in toxicity studies with microalgae. Sci. Total Environ. 2000, 247, 119–126. [Google Scholar] [CrossRef]
- Jamers, A.; Blust, R.; De Coen, W.; Griffin, J.L.; Jones, O.A.H. Copper toxicity in the microalga Chlamydomonas reinhardtii: An integrated approach. Biometals 2013, 26, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Stauber, J.L.; Franklin, N.M.; Adams, M.S. Applications of flow cytometry to ecotoxicity testing using microalgae. Trends Biotechnol. 2002, 20, 141–143. [Google Scholar] [CrossRef]
- Ahmed, N.B.; Masse, S.; Laurent, G.; Piquemal, J.Y.; Yéprémian, C.; Brayner, R.; Coradin, T. Optical microalgal biosensors for aqueous contaminants using organically doped silica as cellular hosts. Anal. Bioanal. Chem. 2017, 410, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Suarez, G.; Santschi, C.; Martin, O.J.F.; Slaveykova, V.I. Biosensor based on chemically-designed anchorable cytochrome c for the detection of H2O2 released by aquatic cells. Biosens. Bioelectron. 2013, 42, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Putzbach, W.; Ronkainen, N.J. Immobilization techniques in the fabrication of nanomaterial-based electrochemical biosensors: A review. Sensors 2013, 13, 4811–4840. [Google Scholar] [CrossRef] [PubMed]
- Koman, V.B.; Santschi, C.; von Moos, N.R.; Slaveykova, V.I.; Martin, O.J.F. Portable oxidative stress sensor: Dynamic and non-invasive measurements of extracellular H2O2 released by algae. Biosens. Bioelectron. 2015, 68, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Suscillon, C.; Velev, O.D.; Slaveykova, V.I. Alternating current-dielectrophoresis driven on-chip collection and chaining of green microalgae in freshwaters. Biomicrofluidics 2013, 7, 024109. [Google Scholar] [CrossRef] [PubMed]
- Bakewell, D.J.; Bailey, J.; Holmes, D. Advancing image quantification methods and tools for analysis of nanoparticle electrokinetics. AIP Adv. 2013, 3, 10201. [Google Scholar] [CrossRef]
- Chuang, C.H.; Ju, J.W.; Huang, Y.W. Enhancing fluorescent response of immunosensing by a dielectrophoresis chip with transparent electrodes and microcavities array. IET Micro Nano Lett. 2013, 8, 659–663. [Google Scholar] [CrossRef]
- Siebman, C.; Velev, O.D.; Slaveykova, V.I. Two-dimensional algal collection and assembly by combining ac-dielectrophoresis with fluorescence detection for contaminant-induced oxidative stress sensing. Biosensors 2015, 5, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Waters, J.C. Accuracy and precision in quantitative fluorescence microscopy. J. Cell Biol. 2009, 185, 1135–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Alargova, R.G.; Kilpatrick, P.K.; Velev, O.D. On-chip dielectrophoretic coassembly of live cells and particles into responsive biomaterials. Langmuir 2010, 26, 3441–3452. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.S.; Tan, J.L.; Voldman, J.; Chen, C.S. Dielectrophoretic registration of living cells to a microelectrode array. Biosens. Bioelectron. 2004, 19, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.M.; Messerli, M.A.; Pethig, R. Spatial manipulation of cells and organelles using single electrode dielectrophoresis. Biotechniques 2012, 52, 39–43. [Google Scholar] [PubMed]
- Ivask, A.; Bondarenko, O.; Jepihhina, N.; Kahru, A. Profiling of the reactive oxygen species-related ecotoxicity of CuO, ZnO, TiO2, silver and fullerene nanoparticles using a set of recombinant luminescent Escherichia coli strains: Differentiating the impact of particles and solubilised metals. Anal. Bioanal. Chem. 2010, 398, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Von Moos, N.; Bowen, P.; Slaveykova, V.I. Bioavailability of inorganic nanoparticles to planktonic bacteria and aquatic microalgae in freshwater. Environ. Sci. Nano 2014, 1, 214–232. [Google Scholar] [CrossRef]
- Von Moos, N.; Maillard, L.; Slaveykova, V.I. Dynamics of sub-lethal effects of nano-cuo on the microalga Chlamydomonas reinhardtii during short-term exposure. Aquat. Toxicol. 2015, 161, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Saison, C.; Perreault, F.; Daigle, J.C.; Fortin, C.; Claverie, J.; Morin, M.; Popovic, R. Effect of core-shell copper oxide nanoparticles on cell culture morphology and photosynthesis (photosystem II energy distribution) in the green alga, Chlamydomonas reinhardtii. Aquat. Toxicol. 2010, 96, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Melegari, S.P.; Perreault, F.; Costa, R.H.R.; Popovic, R.; Matias, W.G. Evaluation of toxicity and oxidative stress induced by copper oxide nanoparticles in the green alga Chlamydomonas reinhardtii. Aquat. Toxicol. 2013, 142, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Soto, P.; Gaete, H.; Hidalgo, M.E. Assessment of catalase activity, lipid peroxidation, chlorophyll-a, and growth rate in the freshwater green algae Pseudokirchneriella subcapitata exposed to copper and zinc. Lat. Am. J. Aquat. Res. 2011, 39, 280–285. [Google Scholar] [CrossRef]
- Han, T.; Kang, S.H.; Park, J.S.; Lee, H.K.; Brown, M.T. Physiological responses of Ulva pertusa and U-armoricana to copper exposure. Aquat. Toxicol. 2008, 86, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, J.; Guo, Y.; Wen, Y.; Liu, J.; Liu, W. Evaluation of the role of the glutathione redox cycle in Cu(II) toxicity to green algae by a chiral perturbation approach. Aquat. Toxicol. 2012, 120, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Perales-Vela, H.V.; Gonzalez-Moreno, S.; Montes-Horcasitas, C.; Canizares-Villanueva, R.O. Growth, photosynthetic and respiratory responses to sub-lethal copper concentrations in Scenedesmus incrassatulus (Chlorophyceae). Chemosphere 2007, 67, 2274–2281. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.J.; Peng, F.Q.; Zhang, L.J.; Ying, G.G. Biosorption of zinc and copper from aqueous solutions by two freshwater green microalgae Chlorella pyrenoidosa and Scenedesmus obliquus. Environ. Sci. Pollut. Res. Int. 2012, 19, 2918–2929. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, S.E.; Juárez, A.B.; Eppis, M.R.; Bianchi, L.; Luquet, C.M.; Ríos de Molina Mdel, C. Oxidative stress and antioxidant defenses in two green microalgae exposed to copper. Ecotoxicol. Environ. Saf. 2009, 72, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Pan, G. Toxicity and bioaccumulation of copper in three green microalgal species. Chemosphere 2002, 49, 471–476. [Google Scholar] [CrossRef]
- Charles, A.L.; Markich, S.J.; Ralph, P. Toxicity of uranium and copper individually, and in combination, to a tropical freshwater macrophyte (Lemna aequinoctialis). Chemosphere 2006, 62, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Prado, R.; Garcia, R.; Rioboo, C.; Herrero, C.; Cid, A. Suitability of cytotoxicity endpoints and test microalgal species to disclose the toxic effect of common aquatic pollutants. Ecotoxicol. Environ. Saf. 2015, 114, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhu, N.; Lavoie, M.; Wang, J.; Qian, H.; Fu, Z. Copper toxicity to Phaeodactylum tricornutum: A survey of the sensitivity of various toxicity endpoints at the physiological, biochemical, molecular and structural levels. Biometals 2014, 27, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Lelong, A.; Jolley, D.F.; Soudant, P.; Hegaret, H. Impact of copper exposure on Pseudo-nitzschia spp. Physiology and domoic acid production. Aquat. Toxicol. 2012, 118, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Hadjoudja, S.; Vignoles, C.; Deluchat, V.; Lenain, J.F.; Le Jeune, A.H.; Baudu, M. Short term copper toxicity on Microcystis aeruginosa and Chlorella vulgaris using flow cytometry. Aquat. Toxicol. 2009, 94, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, M.; Mondolot, L.; Buatois, B.; Andary, C.; Rapior, S.; Kudsk, P.; Mathiassen, S.K.; Ravn, H.W. An easy and rapid method using microscopy to determine herbicide effects in Poaceae weed species. Pest Manag. Sci. 2006, 62, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Nancharaiah, Y.V.; Rajadurai, M.; Venugopalan, V.P. Single cell level microalgal ecotoxicity assessment by confocal microscopy and digital image analysis. Environ. Sci. Technol. 2007, 41, 2617–2621. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Barreiro, O.; Rioboo, C.; Cid, A.; Herrero, C. Atrazine-induced chlorosis in Synechococcus elongatus cells. Arch. Environ. Contam. Toxicol. 2004, 46, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Prado, R.; Rioboo, C.; Herrero, C.; Suarez-Bregua, P.; Cid, A. Flow cytometric analysis to evaluate physiological alterations in herbicide-exposed Chlamydomonas moewusii cells. Ecotoxicology 2012, 21, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Hyun, Y.C.; Min, Y.C.; Min, S.K. Effects of rapid thermal annealing on the optical properties of GaAs quantum dots grown by using the droplet epitaxy method. J. Korean Phys. Soc. 2011, 58, 1324–1329. [Google Scholar] [CrossRef]
- Deng, C.N.; Zhang, D.Y.; Pan, X.L.; Chang, F.Q.; Wang, S.Z. Toxic effects of mercury on PSI and PSII activities, membrane potential and transthylakoid proton gradient in Microsorium pteropus. J. Photochem. Photobiol. B 2013, 127, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Zou, D.; Huang, Y.; Cao, J.; Sheng, G.; Wang, G. Physiological responses of Hizikia fusiformis (Phaeophyta) to mercury exposure. Bot. Mar. 2015, 58, 93–101. [Google Scholar]
- Protopopov, F.F.; Matorin, D.N.; Seifullina, N.K.; Bratkovskaya, L.B.; Zayadan, B.K. Effect of methylmercury on the light dependence fluorescence parameters in a green alga Chlamydomonas moewusii. Microbiology 2015, 84, 822–827. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siebman, C.; Velev, O.D.; Slaveykova, V.I. Probing Contaminant-Induced Alterations in Chlorophyll Fluorescence by AC-Dielectrophoresis-Based 2D-Algal Array. Biosensors 2018, 8, 15. https://doi.org/10.3390/bios8010015
Siebman C, Velev OD, Slaveykova VI. Probing Contaminant-Induced Alterations in Chlorophyll Fluorescence by AC-Dielectrophoresis-Based 2D-Algal Array. Biosensors. 2018; 8(1):15. https://doi.org/10.3390/bios8010015
Chicago/Turabian StyleSiebman, Coralie, Orlin D. Velev, and Vera I. Slaveykova. 2018. "Probing Contaminant-Induced Alterations in Chlorophyll Fluorescence by AC-Dielectrophoresis-Based 2D-Algal Array" Biosensors 8, no. 1: 15. https://doi.org/10.3390/bios8010015
APA StyleSiebman, C., Velev, O. D., & Slaveykova, V. I. (2018). Probing Contaminant-Induced Alterations in Chlorophyll Fluorescence by AC-Dielectrophoresis-Based 2D-Algal Array. Biosensors, 8(1), 15. https://doi.org/10.3390/bios8010015