Cytokeratins Biosensing Using Tilted Fiber Gratings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Tilted Fiber Bragg Gratings Manufacturing
2.3. Optical Interrogation
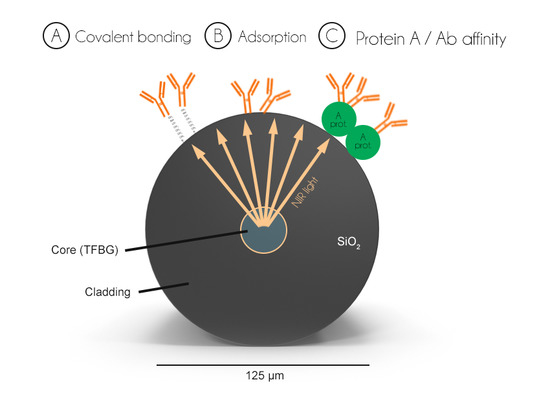
2.4. Biosensor Surface Functionalization
2.5. Data Analysis
3. Results and Discussion
3.1. Stability in Deionized Water and PBS
3.2. Cytokeratin-17 Detection Experiments
3.3. CK17 Specificity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guo, T.; Gonzalez-Vila, A.; Loyez, M.; Caucheteur, C. Plasmonic optical fiber-grating immunosensing: A review. Sensors 2017, 17, 2732. [Google Scholar] [CrossRef] [PubMed]
- Chiavaioli, F.; Baldini, F.; Tombelli, S.; Trono, C.; Giannetti, A. Biosensing with optical fiber gratings. Nanophotonics 2017, 6, 663–679. [Google Scholar] [CrossRef] [Green Version]
- Shevchenko, Y.Y.; Albert, J. Plasmon resonances in gold-coated tilted fiber Bragg gratings. Opt. Lett. 2017, 32, 111–213. [Google Scholar] [CrossRef]
- Daems, D.; Lu, J.; Delport, F.; Mariën, N.; Orbie, L.; Aernouts, B.; Adriaens, I.; Huybrechts, T.; Saeys, W.; Spasic, D.; et al. Competitive inhibition assay for the detection of progesterone in dairy milk using a fiber optic SPR biosensor. Anal. Chim. Acta 2017, 950, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.H.; Sun, T.; Grattan, K.T.V. Optimization of gold-nanoparticle-based fibre surface plasmon resonance (SPR)-based sensors. Sens. Actuator B Chem. 2012, 164, 43–53. [Google Scholar] [CrossRef]
- Springer, T.; Ermani, M.L.; Spackova, B.; Jablonku, J.; Homola, J. Enhancing Sensitivity of Surface Plasmon Resonance Biosensors by Functionalized Gold Nanoparticles: Size Matters. Anal. Chem. 2014, 86, 10350–10356. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Sugimoto, M.; Matsui, Y.; Kondoh, J. Effects of gold film thickness on spectrum profile and sensitivity of a multimode-optical-fiber SPR sensor. Sens. Actuator B Chem. 2008, 132, 26–33. [Google Scholar] [CrossRef]
- Jia, S.; Bian, C.; Sun, J.; Tong, J.; Xia, S. A wavelength-modulated localized surface plasmon resonance (LSPR) optical fiber sensor for sensitive detection of mercury(II) ion by gold nanoparticles-DNA conjugates. Biosens. Bioelectron. 2018, 114, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Park, J.-H.; Byun, J.-Y.; Kim, J.-H.; Kim, M.-G. An optical fiber-based LSPR aptasensor for simple and rapid in-situ detection of ochratoxin A. Biosens. Bioelectron. 2018, 102, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Libralato, G.; Galdiero, E.; Fanga, A.; Carotenuto, R.; de Alterris, E.; Guida, M. Toxicity effects of gunctionalized quantum dots, gold and polystyrene nanoparticles on target aquatic biological models: A review. Molecules 2017, 22, 1439. [Google Scholar] [CrossRef] [PubMed]
- Justino, C.I.L.; Rocha-Santos, T.A.; Duarte, A.C. Review of analytical figures of merit of sensors and biosensors in clinical applications. Trends Anal. Chem. 2010, 29, 1172–1183. [Google Scholar] [CrossRef]
- Chethana, K.; Guru Prasad, A.S.; Omkar, S.N.; Asokan, S. Fiber Bragg grating sensor based device for simultaneous measurement of respiratory and cardiac activities. J. Biophotonics 2017, 10, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Ribaut, C.; Loyez, M.; Larrieu, J.-C.; Chevineau, S.; Lambert, P.; Remmelink, M.; Wattiez, R.; Caucheteur, C. Cancer biomarker sensing using packaged plasmonic optical fiber gratings: Towards in vivo diagnosis. Biosens. Bioelectron. 2017, 92, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Pilla, P.; Trono, C.; Baldini, F.; Chiavaioli, F.; Giordano, M.; Cusano, A. Giant sensitivity of long period gratings in transition mode near the dispersion turning point: An integrated design approach. Opt. Lett. 2012, 37, 4152–4154. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Biswas, P.; Chiavaioli, F.; Dey, T.K.; Basumallick, N.; Trono, C.; Giannetti, A.; Tombelli, S.; Baldini, F.; Bandyopadhyay, S. Long-period fiber grating: A specific design for biosensing applications. Appl. Opt. 2017, 56, 9846–9853. [Google Scholar] [CrossRef] [PubMed]
- Maguis, S.; Laffont, G.; Ferdinand, P.; Carbonnier, B.; Kham, K.; Mekhalif, T.; Millot, M.-C. Biofunctionalized tilted fiber Bragg gratings for label-free immunosensing. Opt. Express 2008, 16, 19049–19062. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, J. Oriented immobilization of proteins on solid supports for use in biosensors and biochips: A review. Microchim. Acta 2016, 183, 1–19. [Google Scholar] [CrossRef]
- Williams, R.A.; Blanch, H.W. Covalent immobilization of protein monolayers for biosensor applications. Biosens. Bioelectron. 1994, 9, 159–167. [Google Scholar] [CrossRef]
- Rusmini, F.; Zhong, Z.; Feijen, J. Protein immobilization strategies for protein biochips. Biomacromolecules 2007, 8, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Bioconjugate Techniques, 3rd ed.; Academic Press: San Diego, CA, USA, 2013. [Google Scholar]
- Jung, Y.; Jeong, J.Y.; Chung, B.H. Recent advances in immobilization methods of antibodies on solid supports. Analyst 2008, 133, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Herr, A.E. Protein immobilization techniques for microfluidic assays. Biomicrofluidics 2013, 7, 41501–41547. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Park, J.M.; Bernhardt, R.; Pyun, J.C. Immunosensor with a controlled orientation of antibodies by using Neutravidin-Protein A complex at immunoaffinity layer. J. Biotechnol. 2006, 126, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Trilling, A.K.; Beelwilder, J.; Zuilof, H. Antibody orientation on biosensor surfaces: A minireview. Analyst 2013, 138, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, M.; Mukai, Y. Immobilization of ultra-thin layer of monoclonal antibody on glass surface. J. Chromatogr. 1991, 566, 361–368. [Google Scholar] [CrossRef]
- Sterzynska, K.; Budna, J.; Frydrych-Tomczak, E.; Hreczycho, G.; Malinska, A.; Maciejewski, H.; Zable, M. Silane-modified surfaces in specific antibody-mediated cell recognition. Folia Histochem. Cytobiol. 2014, 52, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Bremer, M.G.E.G.; Duval, J.; Norde, W.; Lyklema, J. Electrostatic interactions between immunoglobulin (IgG) molecules and charged sobent. Colloids Surf. A 2004, 250, 29–42. [Google Scholar] [CrossRef]
- Albert, J.; Shao, L.-Y.; Caucheteur, C. Tilted fiber Bragg grating sensors. Laser Photonics Rev. 2013, 7, 83–108. [Google Scholar] [CrossRef]
- Chan, C.-F.; Chen, C.; Jafari, A.; Laronche, A.; Thomson, D.J.; Albert, J. Optical fiber refractometer using narrowband cladding-mode resonance shifts. Appl. Opt. 2007, 46, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.J. In-fibre Bragg grating sensors. Meas. Sci. Technol. 1997, 8, 355–375. [Google Scholar] [CrossRef]
- Lépinay, S.; Ianoul, A.; Albert, J. Molecular imprinted polymer-coated optical fiber sensor for the identification of low molecular weight molecules. Talanta 2014, 128, 401–407. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loyez, M.; Albert, J.; Caucheteur, C.; Wattiez, R. Cytokeratins Biosensing Using Tilted Fiber Gratings. Biosensors 2018, 8, 74. https://doi.org/10.3390/bios8030074
Loyez M, Albert J, Caucheteur C, Wattiez R. Cytokeratins Biosensing Using Tilted Fiber Gratings. Biosensors. 2018; 8(3):74. https://doi.org/10.3390/bios8030074
Chicago/Turabian StyleLoyez, Médéric, Jacques Albert, Christophe Caucheteur, and Ruddy Wattiez. 2018. "Cytokeratins Biosensing Using Tilted Fiber Gratings" Biosensors 8, no. 3: 74. https://doi.org/10.3390/bios8030074
APA StyleLoyez, M., Albert, J., Caucheteur, C., & Wattiez, R. (2018). Cytokeratins Biosensing Using Tilted Fiber Gratings. Biosensors, 8(3), 74. https://doi.org/10.3390/bios8030074