The Analysis of Chitosan-Coated Nanovesicles Containing Erythromycin—Characterization and Biocompatibility in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Laboratory Animals
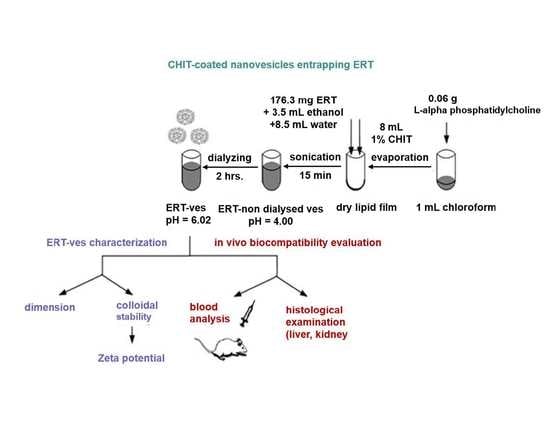
2.3. Preparation of Soft Lipid Vesicles
2.4. Physico-Chemical and Structural Analysis of Lipid Vesicles Loading ERT
2.5. In Vivo Biocompatibility Testing of Lipid Vesicles Loading ERT
- Group 1 (Control): distilled water 0.1 mL/10 g body weight;
- Group 2 (CHIT): chitosan 0.1 mL/10 g body weight;
- Group 3 (ERT): ERT 50 mg/kg body weight;
- Group 4 (ERT-ves): ERT-ves 50 mg/kg body weight.
2.6. Statistical Analysis
3. Results
3.1. pH Value of Lipid Solutions with ERT
3.2. Size Distribution of Lipid Vesicles with ERT
3.3. Zeta Potential of Lipid Vesicles with ERT
3.4. Efficiency of Drug Uptake
3.5. In Vivo Biocompatibility Evaluation Hematological Tests
3.6. Liver Enzyme Activity
3.7. Serum Levels of Urea and Creatinine
3.8. Activity of Immunological Biomarkers
3.9. Hepatic Histopathological Examination
3.10. Renal Histopathological Examination
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Antibiotic Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 28 August 2021).
- Ferreira, M.; Ogren, M.; Dias, J.N.R.; Silva, M.; Gil, S.; Tavares, L.; Aires-da-Silva, F.; Gaspar, M.M.; Aguiar, S.I. Liposomes as antibiotic delivery systems: A promising nanotechnological strategy against antimicrobial resistance. Molecules 2021, 26, 2047. [Google Scholar] [CrossRef] [PubMed]
- Mamun, M.M.; Sorinolu, A.J.; Munir, M.; Vejerano, E.P. Nanoantibiotics: Functions and properties at the nanoscale to combat antibiotic resistance. Front. Chem. 2021, 9, 687660. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Chen, S.; Zhang, J. Bioinspired and biomimetic nanotherapies for the treatment of infectious diseases. Front. Pharmacol. 2019, 10, 751. [Google Scholar] [CrossRef]
- Hallaj-Nezhadi, S.; Hassan, M. Nanoliposome-based antibacterial drug delivery. Drug Deliv. 2015, 22, 581–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez, G.A.; Hosseinidoust, Z. Liposomes for antibiotic encapsulation and delivery. ACS Infect. Dis. 2020, 6, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Muzammil, S.; Hayat, S.; Fakhar-E-Alam, M.; Aslam, B.; Siddique, M.H.; Nisar, M.A.; Saqalein, M.; Atif, M.; Sarwar, A.; Khurshid, A.; et al. Nanoantibiotics: Future nanotechnologies to combat antibiotic resistance. Front. Biosci. 2018, 10, 352–374. [Google Scholar]
- Edson, J.A.; Kwon, J.I. RNAi for silencing drug resistance in microbes toward development of nanoantibiotics. J. Control. Release 2014, 189, 150–157. [Google Scholar] [CrossRef]
- Mititelu-Tartau, L.; Postolache, P.; Dmour, R.; Sindilar, A.; Pinzariu, A.C.; Rozalina, B.; Hilitanu, N.L. New insights into diagnosis and therapeutic modulation of drug-induced hepatotoxicity. Med. Chir. Soc. Med. Nat. Iasi. 2019, 123, 51–56. [Google Scholar]
- Anwekar, H.; Patel, S.; Singhai, A.K. Liposome as drug carriers. Int. J. Pharm. Life. Sci. 2011, 2, 945–951. [Google Scholar]
- Inglut, C.T.; Sorrin, A.J.; Kuruppu, T.; Vig, S.; Cicalo, J.; Ahmad, H.; Huang, H.-C. Immunological and toxicological considerations for the design of liposomes. Nanomaterials 2020, 10, 190. [Google Scholar] [CrossRef] [Green Version]
- Barba, A.A.; Bochicchio, S.; Bertoncin, P.; Lamberti, G.; Dalmoro, A. Coating of nanolipid structures by a novel simil-microfluidic technique: Experimental and theoretical approaches. Coatings 2019, 9, 491. [Google Scholar] [CrossRef] [Green Version]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [Green Version]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Iacob, A.T.; Lupascu, F.G.; Apotrosoaei, M.; Vasincu, I.M.; Tauser, R.G.; Lupascu, D.; Giusca, S.E.; Caruntu, I.-D.; Profire, L. Recent biomedical approaches for chitosan based materials as drug delivery nanocarriers. Pharmaceutics 2021, 13, 587. [Google Scholar] [CrossRef] [PubMed]
- De Leo, V.; Milano, F.; Agostiano, A.; Catucci, L. Recent advancements in polymer/liposome assembly for drug delivery: From Surface modifications to hybrid vesicles. Polymers 2021, 13, 1027. [Google Scholar] [CrossRef]
- Garcia-Fuentes, M.; Prego, C.; Torres, D.; Alonso, M.J. A comparative study of the potential of solid triglyceride nanostructures coated with chitosan or poly(ethylene glycol) as carriers for oral calcitonin delivery. Eur. J. Pharm Sci. 2005, 25, 133–143. [Google Scholar] [CrossRef]
- Jani, P.; Halbert, G.W.; Langridge, J.; Florence, A.T. Nanoparticle uptake by the rat gastrointestinal mucosa: Quantitation and particle size dependency. J. Pharm. Pharmacol. 1990, 42, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J. Surface modification of monodisperse magnetite nanoparticles for improved intracellular uptake to breast cancer cells. J. Colloid Interface Sci. 2005, 283, 352–357. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Butnariu, M.; Rotariu, L.S.; Sytar, O.; Sestito, S.; Rapposelli, S.; Akram, M.; Iqbal, M.; Krishna, A.; et al. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 2021, 21, 318. [Google Scholar] [CrossRef]
- Kukushkina, E.A.; Hossain, S.I.; Sportelli, M.C.; Ditaranto, N.; Picca, R.A.; Cioffi, N. Ag-based synergistic antimicrobial composites. A critical review. Nanomaterials 2021, 11, 1687. [Google Scholar] [CrossRef]
- Pathak, K.; Misra, S.K.; Sehgal, A.; Singh, S.; Bungau, S.; Najda, A.; Gruszecki, R.; Behl, T. Biomedical applications of quaternized chitosan. Polymers 2021, 13, 2514. [Google Scholar] [CrossRef]
- Confederat, L.G.; Tuchilus, C.G.; Dragan, M.; Sha’at, M.; Dragostin, O.M. Preparation and antimicrobial activity of chitosan and its derivatives: A concise review. Molecules 2021, 26, 3694. [Google Scholar] [CrossRef]
- Boroumand, H.; Badie, F.; Mazaheri, S.; Seyedi, Z.S.; Nahand, J.S.; Nejati, M.; Baghi, H.B.; Abbasi-Kolli, M.; Badehnoosh, B.; Ghandali, M.; et al. Chitosan-based nanoparticles against viral infections. Front. Cell Infect. Microbiol. 2021, 11, 643953. [Google Scholar] [CrossRef] [PubMed]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Joni, I.M.; Muchtaridi, M. Chitosan-based nanoparticles of targeted drug delivery system in breast cancer treatment. Polymers 2021, 13, 1717. [Google Scholar] [CrossRef] [PubMed]
- Abdelhakim, M.A.; Radwan, M.S.; Rady, A.H. Chitosan nanoparticles as hepato-protective agent against alcohol and fatty diet stress in rats. Biochem. Int. 2017, 4, 5–10. [Google Scholar]
- Santhosh, S.; Sini, T.K.; Anandan, R.; Paruthapara, M. Hepatoprotective activity of chitosan against isoniazid and rifampicin-induced toxicity in experimental rats. Eur. J. Pharmacol. 2007, 572, 69–73. [Google Scholar] [CrossRef]
- Sahu, A.K.; Kumar, T.; Jain, V. Formulation optimization of erythromycin solid lipid nanocarrier using response surface methodology. BioMed Res. Int. 2014, 2014, 689391. [Google Scholar] [CrossRef]
- Wróblewska, M.; Winnicka, K. The effect of cationic polyamidoamine dendrimers on physicochemical characteristics of hydrogels with erythromycin. Int. J. Mol. Sci. 2015, 16, 20277–20289. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Pan, C.; Lu, Y.; Gao, Y.; Liu, W.; Yin, P.; Yu, X. Combination of erythromycin and curcumin alleviates Staphylococcus aureus induced osteomyelitis in rats. Front. Cell Infect. Microbiol. 2017, 7, 379. [Google Scholar] [CrossRef]
- Husada, D.; Soegianto, S.D.P.; Kurniawati, I.S.; Hendrata, A.P.; Irawan, E.; Kartina, L.; Puspitasari, D.; Basuki, P.S.; Ismoedijanto. First-line antibiotic susceptibility pattern of toxigenic Corynebacterium diphtheriae in Indonesia. BMC Infect. Dis. 2019, 19, 1049. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Gu, D.; Hou, G. Erythromycin attenuates metalloprotease/anti-metalloprotease imbalance in cigarette smoke-induced emphysema in rats via the mitogen-activated protein kinase/nuclear factor-κB activation pathway. Mol. Med. Rep. 2017, 15, 2983–2990. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Qiu, S.L.; Tang, Q.Y.; Zhou, X.; Zhang, J.Q.; He, Z.Y.; Bai, J.; Li, M.H.; Deng, J.M.; Liang, Y.; et al. Erythromycin suppresses neutrophil extracellular traps in smoking-related chronic pulmonary inflammation. Cell Death Dis. 2019, 10, 678. [Google Scholar] [CrossRef]
- Bhadra, S.; Prajapati, A.B.; Bhadra, D. Development of pH sensitive polymeric nanoparticles of erythromycin stearate. J. Pharm. Bioallied Sci. 2016, 8, 135–140. [Google Scholar] [CrossRef] [PubMed]
- European Union. DIRECTIVE 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes; European Union: Brussels, Belgium, 2010; Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF (accessed on 10 July 2021).
- Garlea, A.; Popa, M.I.; Pohoata, V.; Melnig, V. Ibuprofen/ketoprofen entrapment in chitosan based vesicle carrier. Rom. J. Biophys. 2007, 17, 157–168. [Google Scholar]
- Bindar, D.; Garlea, A.; Tartau, L.; Chiriac, A.P.; Nita, L.; Melnig, V. Effect of acetaminophen soft matter vesicles carrier in a somatic pain model in mice. Ann. Rom. Soc. Cell Biol. 2009, 14, 256–260. [Google Scholar]
- Parasuraman, S.; Raveendran, R.; Kesavan, R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 2010, 1, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Diehl, K.-H.; Hull, R.; Morton, D.; Pfister, R.; Rabemampianina, Y.; Smith, D.; Vidal, J.M.; van de Vorstenbosch, C. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J. Appl. Toxicol. 2001, 21, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Suckow, M.; Danneman, P.; Brayton, C. The Laboratory Mouse; CRC Press LLC: Boca Raton, FL, USA, 2001. [Google Scholar]
- Roy, J.; Salaün, F.; Giraud, S.; Ferri, A. Solubility of chitin: Solvents, solution behaviors and their related mechanisms. In Solubility of Polysaccharides; IntechOpen: London, UK, 2017. [Google Scholar]
- Pignatello, R.; Fuochi, V.; Petronio, P.G.; Greco, A.; Furneri, P.M. Formulation and characterization of erythromycin–loaded solid lipid nanoparticles. Biointerface Res. Appl. Chem. 2017, 7, 2145–2150. [Google Scholar]
- Dhillon, P.; Mirza, M.A.; Anwer, M.K.; Alshetaili, A.S.; Alshahrani, S.M.; Iqbal, Z. Development and optimization of erythromycin-loaded lipid-based gel by Taguchi design: In vitro characterization and antimicrobial evaluation. Braz. J. Pharm. Sci. 2019, 55, e17395. [Google Scholar] [CrossRef] [Green Version]
- Gbian, D.L.; Omri, A. The impact of an efflux pump inhibitor on the activity of free and liposomal antibiotics against Pseudomonas aeruginosa. Pharmaceutics 2021, 13, 577. [Google Scholar] [CrossRef]
- Stuhne-Sekalec, L.; Stanacev, N.Z.; Djokic, S. Liposomes as carriers of macrolides: Preferential association of erythromycin A and azithromycin with liposomes of phosphatidylglycerol containing unsaturated fatty acid(s). J. Microencapsul. 1991, 8, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Solleti, V.S.; Alhariri, M.; Halwani, M.; Omri, A. Antimicrobial properties of liposomal azithromycin for Pseudomonas infections in cystic fibrosis patients. J. Antimicrob. Chemother. 2015, 70, 784–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunardi, C.N.; Gomes, A.J.; Rocha, F.S.; De Tommaso, J.; Patience, G.S. Experimental methods in chemical engineering: Zeta potential. Can. J. Chem. Eng. 2021, 99, 627–639. [Google Scholar] [CrossRef]
- Midekessa, G.; Godakumara, K.; Ord, J.; Viil, J.; Lättekivi, F.; Dissanayake, K.; Kopanchuk, S.; Rinken, A.; Andronowska, A.; Bhattacharjee, S.; et al. Zeta potential of extracellular vesicles: Toward understanding the attributes that determine colloidal stability. ACS Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef]
- Larsson, M.; Hill, A.; Duffy, J. Suspension stability; why particle size, zeta potential and rheology are important. Annu. Trans. Nord. Rheol. Soc. 2012, 12, 209–214. [Google Scholar]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B 2019, 9, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Sebaaly, C.; Trifan, A.; Sieniawska, E.; Greige-Gerges, H. Effect of chitosan coating on liposome characteristics: An emphasis on bioactive compounds and essential oils: A review. Processes 2021, 9, 445. [Google Scholar] [CrossRef]
- Ladiè, R.; Cosentino, C.; Tagliaro, I.; Antonini, C.; Bianchini, G.; Bertini, S. Supramolecular structuring of hyaluronan-lactose-modified chitosan matrix: Towards high-performance biopolymers with excellent biodegradation. Biomolecules 2021, 11, 389. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wang, S.; Wang, Y.; Wanga, X.; Wang, Q.; Chen, M. Advances in self-assembled chitosan nanomaterials for drug delivery. Biotechnol. Adv. 2014, 32, 1301–1316. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.; Sun, Y.; Cui, H. Characterization of chrysanthemum essential oil triple-layer liposomes and its application against Campylobacter jejuni on chicken. LWT 2019, 107, 16–24. [Google Scholar] [CrossRef]






| Solution | pH |
|---|---|
| Erythromicyn vesicles (ERT) | 6.12 |
| Erythromycin vesicles in chitosan (before dialysis) (ERT-non dialysed-ves) | 4.00 |
| Erythromycin vesicles in chitosan (after dialysis) (ERT-ves) | 6.02 |
| Point in Time | GR (mil/μL) | Hb (g/mL) | Ht (%) | |
|---|---|---|---|---|
| Control | 24 h | 8.50 ± 1.05 | 12.67 ± 2.58 | 43.67 ± 2.73 |
| 7 days | 9.77 ± 0.14 | 15.12 ± 0.57 | 41.48 ± 1.32 | |
| CHIT | 24 h | 9.34 ± 0.57 | 15.41 ± 0.25 | 41.50 ± 0.14 |
| 7 days | 9.79 ± 0.10 | 15.67 ± 0.38 | 42.01 ± 0.57 | |
| ERT | 24 h | 9.80 ± 0.73 | 15.37 ± 1.58 | 41.52 ± 0.70 |
| 7 days | 9.82 ± 0.25 | 15.40 ± 0.42 | 41.55 ± 2.38 | |
| ERT-ves | 24 h | 9.59 ± 1.28 | 15.24 ± 0.73 | 40.69 ± 3.14 |
| 7 days | 9.64 ± 0.16 | 15.23 ± 0.42 | 40.72 ± 0.67 |
| Point in Time | Leucokyte Formula | |||||
|---|---|---|---|---|---|---|
| % | ||||||
| PMN | Ly | E | M | B | ||
| Control | 24 h | 19.72 ± 2.42 | 77.83 ± 4.59 | 0.47 ± 0.26 | 1.78 ± 1.23 | 0.20 ± 0.07 |
| 7 days | 19.73 ± 2.95 | 77.86 ± 3.72 | 0.52 ± 0.48 | 2.05 ± 1.81 | 0.22 ± 0.13 | |
| CHIT | 24 h | 18.74 ± 2.87 | 79.26 ± 4.42 | 0.57 ± 0.52 | 1.23 ± 1.17 | 0.20 ± 0.03 |
| 7 days | 18.73 ± 1.91 | 80.88 ± 1.82 | 0.72 ± 0.85 | 1.45 ± 1.22 | 0.20 ± 0.17 | |
| ERT | 24 h | 19.44 ± 3.23 | 77.33 ± 4.86 | 0.53 ± 0.42 | 2.45 ± 1.07 | 0.25 ± 0.07 |
| 7 days | 19.35 ± 3.74 | 76.05 ± 5.42 | 0.57 ± 0.71 | 2.98 ± 1.25 | 0.28 ± 0.20 | |
| ERT-ves | 24 h | 18.75 ± 3.23 | 78.97 ± 4.59 | 0.56 ± 0.59 | 1.47 ± 0.26 | 0.25 ± 0.13 |
| 7 days | 18.77 ± 2.42 | 79.45 ± 2.84 | 0.55 ± 0.52 | 0.95 ± 0.52 | 0.27 ± 0.23 | |
| Point in Time | ALT (U/mL) | AST (U/mL) | LDH (U/L) | |
|---|---|---|---|---|
| Control | 24 h | 40.30 ± 3.19 | 120.50 ± 19.29 | 1254.17 ± 165.33 |
| 7 days | 43.33 ± 3.62 | 119.00 ± 18.17 | 1250.88 ± 167.04 | |
| CHIT | 24 h | 42.17 ± 5.21 | 159.33 ± 35.20 | 1311.27 ± 249.19 |
| 7 days | 46.83 ± 6.43 | 161.17 ± 37.62 | 1904.33 ± 264.80 ** | |
| ERT | 24 h | 39.30 ± 6.17 | 120.50 ± 18.43 | 1302.13 ± 101.33 |
| 7 days | 37.8 ± 4.38 | 117.17 ± 12.29 | 1689.00 ± 96.49 ** | |
| ERT-ves | 24 h | 39.67 ± 5.04 | 140.50 ± 28.33 | 1398.17 ± 185.43 |
| 7 days | 41.00 ± 5.33 | 142.00 ± 31.71 | 1407.67 ± 294.90 |
| Point of Time | Urea (mg/mL) | Creatinine (mg/dL) | |
|---|---|---|---|
| Control | 24 h | 47.83 ± 6.43 | 0.38 ± 0.02 |
| 7 days | 45.00 ± 5.87 | 0.39 ± 0.02 | |
| CHIT | 24 h | 46.67 ± 1.37 | 0.38 ± 0.02 |
| 7 days | 42.33 ± 4.03 | 0.37 ± 0.03 | |
| ERT | 24 h | 45.83 ± 1.94 | 0.38 ± 0.01 |
| 7 days | 42.67 ± 8.31 | 0.37 ± 0.01 | |
| ERT-ves | 24 h | 49.33 ± 3.14 | 0.37 ± 0.01 |
| 7 days | 50.67 ± 2.07 | 0.37 ± 0.03 |
| Point of Time | OC (Bacterial Colonies/mL) | PC (Bacterial Colonies/mL) | BC (Bacterial Colonies/mL) | |
|---|---|---|---|---|
| Control | 24 h | 808.33 ± 25.00 | 531.50 ± 15.60 | 715.67 ± 10.76 |
| 7 days | 787.17 ± 30.80 | 529.67 ± 13.02 | 712.33 ± 9.69 | |
| CHIT | 24 h | 805.40 ± 29.73 | 527.43 ± 14.48 | 713.55 ± 9.80 |
| 7 days | 794.43 ± 29.58 | 530.67 ± 14.55 | 719.40 ± 11.48 | |
| ERT | 24 h | 811.21 ± 29.47 | 530.83 ± 15.05 | 715.33 ± 11.58 |
| 7 days | 791.17 ± 29.53 | 532.55 ± 14.03 | 717.67 ± 13.47 | |
| ERT-ves | 24 h | 806.67 ± 29.76 | 530.60 ± 14.58 | 717.55 ± 16.33 |
| 7 days | 799.17 ± 30.11 | 533.21 ± 13.24 | 719.17 ± 17.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilițanu, L.N.; Mititelu-Tarțău, L.; Popa, G.E.; Buca, B.R.; Pavel, L.L.; Pelin, A.-M.; Meca, A.-D.; Bogdan, M.; Pricop, D.A. The Analysis of Chitosan-Coated Nanovesicles Containing Erythromycin—Characterization and Biocompatibility in Mice. Antibiotics 2021, 10, 1471. https://doi.org/10.3390/antibiotics10121471
Hilițanu LN, Mititelu-Tarțău L, Popa GE, Buca BR, Pavel LL, Pelin A-M, Meca A-D, Bogdan M, Pricop DA. The Analysis of Chitosan-Coated Nanovesicles Containing Erythromycin—Characterization and Biocompatibility in Mice. Antibiotics. 2021; 10(12):1471. https://doi.org/10.3390/antibiotics10121471
Chicago/Turabian StyleHilițanu, Loredana Nicoleta, Liliana Mititelu-Tarțău, Grațiela Eliza Popa, Beatrice Rozalina Buca, Liliana Lăcrămioara Pavel, Ana-Maria Pelin, Andreea-Daniela Meca, Maria Bogdan, and Daniela Angelica Pricop. 2021. "The Analysis of Chitosan-Coated Nanovesicles Containing Erythromycin—Characterization and Biocompatibility in Mice" Antibiotics 10, no. 12: 1471. https://doi.org/10.3390/antibiotics10121471
APA StyleHilițanu, L. N., Mititelu-Tarțău, L., Popa, G. E., Buca, B. R., Pavel, L. L., Pelin, A. -M., Meca, A. -D., Bogdan, M., & Pricop, D. A. (2021). The Analysis of Chitosan-Coated Nanovesicles Containing Erythromycin—Characterization and Biocompatibility in Mice. Antibiotics, 10(12), 1471. https://doi.org/10.3390/antibiotics10121471